
Journal of Clinical Chemistry and Laboratory Medicine
Open Access
ISSN: 2736-6588

ISSN: 2736-6588
Research Article - (2022)Volume 5, Issue 8
Background: Systemic Lupus Erythematosus (SLE) is one of the autoimmune disorders affecting many organs and systems with heterogeneity in clinical manifestations and disease course. Cytokines may play a role in the pathogenesis of the disease.
Objectives: To assess the possible role of the polymorphisms of IL-18 and IL-27 genes in SLE development.
Subjects and methods: A case control study was conducted on 120 patients with SLE and 100 ages-matched healthy individuals considered the control group. The IL-27-924A/G and IL-18-607C/A polymorphisms by RFLP-PCR.
Results: For IL-27 924A/G gene polymorphism, The AA genotype was more frequent in SLE group in comparison to the healthy individuals (P=0.04, OR (95% CI)=2.3(1-5.4). On the other hand, AG genotype frequency was higher in the control group (p=0.03, OR (95%CI)=0.4(0.16-0.9). In addition, we observed that AA genotype and A allele were associated with lupus nephritis. Regarding IL-18-607C/A polymorphism, we did not found any variation in the frequency of genotypes and alleles between the studied groups. There was relationship between A allele and lupus nephritis.
Conclusion: We concluded that the susceptibility to SLE was increased with IL-27-924 AA genotype, while the AG genotype may have a protective role in the occurrence of SLE however IL-18-607C/A polymorphism has no role the disease development but AA allele might increase the incidence of lupus nephritis.
Lupus; Gene polymorphism; Autoimmune disease; Genes; Immune response
Systemic Lupus Erythematosus (SLE) is an autoimmune disease of unknown cause affecting many systems in the body and manifested by production of many autoantibodies, stimulation of the complement system, and deposition of immune complex which leads to various clinical pictures and tissue destruction [1]. As the real cause of SLE still unclear, genetic susceptibility, hormonal and environmental factors are identified to have fundamental roles in the pathophysiology of the disease [2].
Genetic studies on a large scale have recognized about 50 genetic loci might be involved in lupus susceptibility; these loci include the genetic variants associated with the different phases of the immune response [3].
Genes which encode cytokines that affecting the differentiation of CD4+ T cell are target factors that may affect not only the pathogenesis but also the course of SLE [4]. The cytokines, IL-12 and IL-27, trigger organ damage in SLE as they have pivotal roles in the inflammatory process by stimulation the early stages of Th1 cell differentiation and increase the activities of both natural killer and cytotoxic T lymphocytes cells [5]. Human gene encoding IL27 is located on chromosome 16p11. IL-27 is a protein is secreted a dimmer consists of 2 monomers, EB3 and P28. This cytokine has roles in Th cell responses and inflammation [6].
Cytokines related to Th1 and Th2 immune response could be involved in the disease pathogenesis as the peripheral blood cells of these patients show diminished secretion of the Th1 cytokines; IL-2, interferon-gamma (IFN-γ), Tumor Necrosis Factor-alpha (TNFa) and IL-12 as well as increased production of the Th2 cytokines; IL-4 and IL-10 [7]. IL-18 is considered Th1 cytokine due to its property to stimulate IFN-γ [8].
IL-18 gene is situated at q22.2-22.3 of chromosome 11, spanning six exons and five introns. Previous reports have recognized that polymorphisms in IL-18 could affect the expression of IL-18, which in turn affecting the level of TFN-γ and TH1 immunological response [9]. There are two common polymorphisms in the promoter of the IL- 18 gene,-137G/C and -607C/A, might affecting the function and activity of IL-18 protein [10]. The purpose of this research is to study the possible association of IL-27-924A/G and IL-18-607C/A genes polymorphisms and susceptibility to SLE in Egyptian population.
Subjects and methods
Subject: A case control study was conducted on 120 SLE whom were recruited from OPC of Rheumatology & Immunology Unit, Internal Medicine Department, Mansoura University Hospital, Egypt. The diagnosis of the patients was based on the criteria of American College of Rheumatology [11]. The clinical manifestation of SLE in the patients group includes central nervous system, vascular, renal, musculoskeletal, arthritis, and serosal, dermal and hematologic components were evaluated. One hundred healthy individuals have no personal or family history of autoimmune diseases were represented the control group. Familial, epidemiological and clinical data from individuals were obtained by questionnaires and medical records. The study was done with the agreement of Mansoura University Ethical Committee (code no: R/18.02.60) and in accordance with the General Assembly of the World Medical Association Declaration of Helsinki .Written agreement was taken from each participant.
Methods: One ml blood sample was collected from each participant in EDTA tubes for fresh DNA extraction and DNA was stored at- 80º C until the later use for genotyping of IL-27-924A/G and IL-18-607C/A genes polymorphisms by Polymerase Chain Reaction (PCR) and Restriction Fragment Length Polymorphism (RFLP).
Total genomic DNA was isolated from blood by DNA extraction kit (Qiagen GmbH, Cat No.51104, Hiden, Germany). PCR was used for IL-27− 924 A/G gene as described by Paradowska- Gorycka et al. [12]. Two primers were used: F5`-CTGATCCTGACCTCACTCAACGC-3`, R5`CTGACTGGGACTGGGACTCAGC-3`. The temperature program was: Initial denaturation for 8 min at 95 °C, then 35 cycles of: 45 s at 94°C, 45s at 55 °C and 45s at 72 °C , followed for 8 min at 72 °C as a final extension. The PCR product was 119 bp length. PCR products were subjected to 1 U of BstU I restriction enzyme (Thermo scientific, USA), the products were detected using 3% agarose gel electrophoresis and compared with 50 bp DNA Marker. The A allele gave 119bp fragment, while the G allele gave 95 and 24bp fragments (Figure 1).
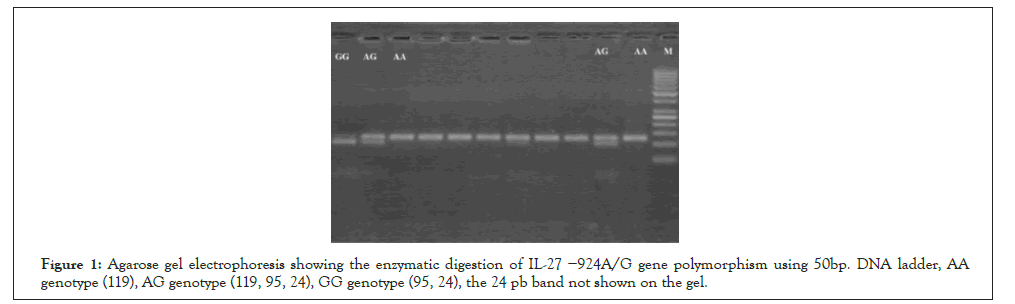
Figure 1: Agarose gel electrophoresis showing the enzymatic digestion of IL-27 −924A/G gene polymorphism using 50bp. DNA ladder, AA genotype (119), AG genotype (119, 95, 24), GG genotype (95, 24), the 24 pb band not shown on the gel.
Regarding IL-18-607C/A polymorphism, Two primers were used for amplification of a 137 Base Pair (bp) fragment included in the -607C/A of the IL-18 gene polymorphism [13]. 5`-CCCTCTCCCCAAGCTTACTT-3`and R 5`-TTCAGTGGAACAG GAGTCCA-3`. DNA was initially denatured for 5 min at 94 °C, then 35 cycles of 94 °C for 55 s, 62 °C for 48 s, 72 °C for 55 s. DNA amplification was completed at 72°C for 7 min as a final extension. The PCR product was 137 bp. The restriction enzyme used was MseI (Thermo scientific, USA). Allele C produced 137bp band, while the A allele gave 91 and 46bp fragments (Figure 2).
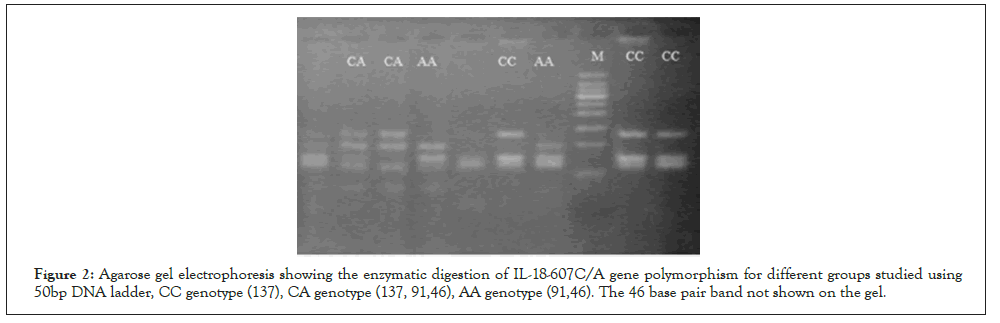
Figure 2: Agarose gel electrophoresis showing the enzymatic digestion of IL-18-607C/A gene polymorphism for different groups studied using 50bp DNA ladder, CC genotype (137), CA genotype (137, 91,46), AA genotype (91,46). The 46 base pair band not shown on the gel.
Statistical Analysis
Excel program and SPSS version 21 was used to analyze the resulting data. For analysis of quantitative data, Mann-Whitney test was used to compare two groups, the Wilcoxon test was used to compare the two paired groups and for comparison of more than two groups, Kruskal Wallis test was used. To compare qualitative data, c2 test was used. P<0.05 was considered significant at 95% confidence interval. The Hardy-Weinberg Equilibrium was used for testing the frequency of genotypes and alleles in both groups.
The patient group has higher levels of ANA, anti-dsDNA and ESR and significant lower levels of C3, C4 and Hemoglobin. No discriminations between the studied groups regarding age, sex, BMI and CRP were observed (Table 1).
| Parameters | Control (n= 100) | SLE (n=120) | P-value |
|---|---|---|---|
| Age | 33.40 ± 5.76 | 32.23 ± 7.81 | NS |
| Gender(female/male) | 98/2 | 116/4 | NS |
| Disease duration | -------- | 5.79 ± 2.01 | ----- |
| BMI(kg/m2) | 27.90 ± 4.60 | 28.68 ± 5.09 | NS |
| ANA(IU/ml) | 0.76 ± 0.19 | 3.46 ± 2.38 | 0 |
| Ads DNA(IU/ml) | 8.57 ± 1.77 | 104.67 ± 30.53 | 0 |
| CRP(mg/dl) | 4.01 ± 1.52 | 4.23 ± 3.54 | NS |
| ESR(mm/hour) | 10.29 ± 3.62 | 57.92 ± 24.14 | 0 |
| C4(mg/dl) | 18.45 ± 5.86 | 4.36 ± 2.22 | 0 |
| C3(mg/dl) | 153.56 ± 27.58 | 17.40 ± 5.35 | 0 |
| SLEDAI | ------- | 9.97 ± 4.55 | ----- |
| Hb | 12.34 ± 0.82 | 10.92 ± 1.71 | 0.001 |
Note: BMI: Body Mass Index; ANA: Anti- Nuclear Antibody; AdsDNA= Anti-Double Stranded DNA; CRP= C-Reactive Protein; ESR= Erythrocyte Sedimentation Rate; C3= Complement 3; C4= Complement 4; SLEDAI=Systemic Lupus Disease Activity Index; Hb= Hemoglobin
Genotyping of the study population
The distribution of genotypes and alleles was in Hardy- Weinberg equilibrium (P>0.05). PCR determination of IL-27-924A/G gene polymorphism revealed that, there was increased incidence of AA genotype in the patients group when compared with the controls (P=0.04, OR (95%CI)=2.3(1- 5.4). On the other hand, the heterozygous AG genotype was frequent in the control group (p=0.03, OR (95%CI)=0.4(0.16- 0.9). The result of PCR detection of the IL-18 607C/A gene polymorphism showed no significant variations in the frequency of genotypes (CC, CA and AA) and alleles (C and A) between patients and normal individuals (Table 2).
| Polymorphism | Controls (n=100) | Patients (n = 120 ) | P value | OR (95 % CI) | |
|---|---|---|---|---|---|
| IL-27−924A/G | Co-dominant model | ||||
| AA | 12 (12%) | 28(23.3%) | ------ | 1 (Ref) | |
| AG | 63 (63%) | 60 (50%) | 0.03 | 0.4(0.16-0.9) | |
| GG | 25 (25%) | 32 (26.7%) | 0.16 | 0.5(0.18-1.3) | |
| Dominant Model | |||||
| AG+GG | 88 (88%) | 88 (73.3%) | ------ | 1 (Ref) | |
| AA | 12 (12%) | 32 (26.7%) | 0.04 | 2.3(1-5.4) | |
| Recessive Model | |||||
| AG+AA | 75 (75%) | 92 (76.7%) | -0.57 | 1 (Ref) | |
| GG | 25 (25%) | 28 (23.3%) | 0.8 (0.4-1.7) | ||
| Allele | |||||
| A | 87 (43.5%) | 124 | 0.14 | 1 (Ref) | |
| G | 113 (56.5%) | 116 | 0.4(0.46-1.1) | ||
Note: Odds ratio: 95% confidence interval (OR 95 % CI); Ref: Reference
The AA variant was linked with high levels of CRP (P=0.004) an d no differences in the other parameters between the three genotypes. The incidence of lupus nephritis and levels of CRP, ESR were high in SLE patients with A allele (Table 3).
| Polymorphism | Controls (n= 100) | Patients (n = 120 ) | P value | OR (95 % CI) | |
|---|---|---|---|---|---|
| IL-18 −607C/A | Co-dominant model | ||||
| CC | 28 (28%) | 28 (23.3%) | 0.52 | 1 (Ref) | |
| AC | 47 (47%) | 58 (48.3%) | 0.41 | 1.2(0.6-2.3) | |
| AA | 25 (25%) | 34 (28.3%) | 1.3(0.5-2.8) | ||
| Dominant Model AC+CC | |||||
| AA | 75 (75%) | 92 (76.7%) | 0.57 | 1 (Ref) | |
| 25 (25%) | 28 (23.3%) | 1.1(0.6-2.1) | |||
| Recessive Model AC+AA | |||||
| CC | 72 (72%) | 92 (76.7%) | -0.42 | 1 (Ref) | |
| 28 (28%) | 28 (23.3%) | 0.7 (0.4-1.4) | |||
| Allele | |||||
| C | 103 (51.5%) | 114 (47.5) | 0.4 | 1 (Ref) | |
| A | 97 (48.5%) | 126 (52.5) | 1.1(0.8-1.7) | ||
Table 3: Genotypes and alleles distribution of the IL-18 gene polymorphisms in SLE patients and controls.
AA variant was linked with high levels of CRP (P=0.001) and no variations in the other parameters between the three genotypes. The incidence of lupus nephritis and levels of CRP were increased in SLE patients with A allele (Table 4 and Table 5).
| Clinical and laboratory data | AA genotype (n=32) | AG genotype (n=60) | GG genotype (n=28) | P-value | A allele (n=124) | G allele (n=116) | P-value |
|---|---|---|---|---|---|---|---|
| Age(year) | 32.1 ± 8.8 | 32.9 ± 7.8 | 31.0 ± 6.7 | NS | 32.3 ± 8.4 | 38.3 ± 6.1 | NS |
| Gender (femalemale) | 33/1 | 57/3 | 25-Jan | NS | 119/5 | 111/5 | NS |
| Disease duration(year) | 6.1 ± 3.4 | 5.9 ± 4.0 | 5.0 ± 2.0 | NS | 5.9 ± 3.5 | 5.4 ± 2.8 | NS |
| BMI(kg/m2) | 28.2 ± 5.0 | 29.0 ± 3.8 | 29.3 ± 3.2 | NS | 27.9 ± 5.1 | 28.2 ± 4.8 | NS |
| ANA (IU/ml) | 3.4 ± 2.5 | 4.0 ± 2.4 | 3.1 ± 2.1 | NS | 3.6 ± 2.4 | 3.5 ± 2.4 | NS |
| Ads DNA (IU/ml) | 101.4 ± 27.8 | 107.0 ± 32.1 | 109.1 ± 32.5 | NS | 104.9 ± 28.8 | 104.0 ± 29.9 | NS |
| CRP (mg/dl) | 5.9 ± 3.4 | 3.6 ± 3.1 | 3.4 ± 2.6 | 0.004 | 4.9 ± 4.0 | 3.7 ± 2.9 | 0.01 |
| ESR (mm/hour) | 58.2 ± 28.6 | 50.2 ± 34.7 | 48.3 ± 30.1 | NS | 54.8 ± 31.7 | 47.5 ± 32.1 | 0.03 |
| C4 (mg/dl) | 4.5 ±1.4 | 4.4 ± 2.4 | 3.9 ± 1.6 | NS | 3.9 ±1.58 | 4.1 ± 1.9 | NS |
| C3 (mg/dl) | 16.5 ± 5.5 | 17.7 ± 5.5 | 17.7 ± 5.0 | NS | 17.0 ± 5.1 | 17.3 ± 5.2 | NS |
| Hb (gm/dl) | 10.5 ± 1.1 | 10.8 ± 1.2 | 10.4 ± 1.4 | NS | 10.6 ± 1.2 | 10.6 ± 1.3 | NS |
| SLEDA INDEX | 9.4 ± 4.3 | 10.6 ± 4.8 | 10.5 ± 4.8 | NS | 10.2 ± 4.1 | 9.8 ± 4.1 | NS |
| Nephritis (Number) | 15 | 20 | 11 | NS | 53 | 41 | 0.002 |
| Skin manifestation (Number) | 34 | 60 | 26 | NS | 128 | 116 | NS |
| Arthritis (Number) | 19 | 30 | 15 | NS | 69 | 63 | NS |
| Neuropsychtric (Number) | 1 | 3 | 1 | NS | 4 | 4 | NS |
| Serositis (Number) | 3 | 9 | 5 | NS | 16 | 18 | NS |
Note: BMI: Body Mass Index; ANA: Anti- Nuclear Antibody; Ads DNA: Anti-Double Stranded DNA; CRP:C Reactive Protein; ESR: Erythrocyte Sedimentation Rate; C3: Complement 3; C4: Complement 4; SLEDAI: Systemic Lupus Disease Activity Index; Hb: Hemoglobin
Table 4: Steps to reach pain 5 and 2 after SLSD test.
| Clinical and laboratory data | AA genotype (n=34) | AC genotype (n=58) | CC genotype (n=28) | P-value | A allele (n=126) | C allele (n=114) | P-value |
|---|---|---|---|---|---|---|---|
| Age(year) | 32.1 ± 8.2 | 32.3 ± 7.9 | 32.0 ± 7.5 | NS | 32.5 ± 8.0 | 31.7 ± 7.6 | NS |
| Gender(F/M) | 33/1 | 555/3 | 27-Jan | NS | 121/5 | 109/5 | NS |
| Disease duration(y) | 6.2 ± 4.0 | 6.0 ± 3.4 | 4.9 ± 2.3 | NS | 6.0 ± 3.5 | 5.5 ± 2.9 | NS |
| BMI(kg/m2) | 28.0 ± 5.1 | 29.4 ± 4.3 | 28.8 ± 4.0 | NS | 28.2 ± 4.9 | 28.5 ± 4.8 | NS |
| ANA(IU/ml) | 3.7 ± 2.1 | 3.6 ± 2.9 | 3.9 ± 2.7 | NS | 3.6 ± 2.5 | 3.2 ± 2.3 | NS |
| Ads DNA(IU/ml) | 109.0 ± | 102.4 ± | 104.1 ± | NS | 105.6 ± 30.0 | 103.0 ± 28.3 | NS |
| 32.3 | 29 | 30.1 | |||||
| CRP(mg/dl) | 6.0 ± 4.5 | 3.6 ± 3.1 | 3.4 ± 2.3 | 0.001 | 4.5 ± 4.0 | 3.6 ± 2.6 | 0.03 |
| ESR(mm/hour) | 53.8 ± | 51.9 ± | 50.3 ± | NS | 53.8 ± 31.7 | 49.7 ± 33.1 | NS |
| 29.2 | 34.6 | 30.9 | |||||
| C4(mg/dl) | 4.4 ± 2.2 | 4.6 ± 2.3 | 3.8 ± 2.0 | NS | 4.2 ± 2.0 | 4.0 ± 2.0 | NS |
| C3(mg/dl) | 16.4 ± 5.1 | 17.5 ± 5.5 | 18.3 ± 5.5 | NS | 16.7 ± 4.9 | 17.8 ± 5.4 | NS |
| Hb(gm/dl) | 10.2 ± 1.1 | 10.1 ± 1.3 | 10.5 ± 1.5 | NS | 10.2 ± 1.5 | 10.5 ± 1.4 | NS |
| SLEDA INDEX | 10.8 ± 4.9 | 9.2 ± 4.2 | 10.6 ± 4.6 | NS | 10.2 ± 4.7 | 9.9 ± 4.4 | NS |
| Nephritis (Number) | 17 | 18 | 11 | NS | 53 | 39 | 0.001 |
| Skin manifestation (Number) | 30 | 58 | 32 | NS | 126 | 114 | NS |
| Arthritis(Number) | 17 | 28 | 19 | NS | 63 | 65 | NS |
| Neuropsychtric (Number) | 1 | 3 | 1 | NS | 4 | 4 | NS |
| Serositis (Number) | 4 | 7 | 6 | NS | 17 | 17 | NS |
Note: BMI: Body Mass Index; ANA: Anti- Nuclear Antibody; Ads DNA: Anti-Double Stranded DNA; CRP:C Reactive Protein; ESR: Erythrocyte Sedimentation Rate; C3: Complement 3; C4: Complement 4; SLEDAI: Systemic Lupus Disease Activity Index; Hb: Hemoglobin
Table 5: Clinical and laboratory data of SLE patients in different genotypes and alleles of IL-18-607C/A gene.
SLE is a heterogeneous disorder manifested by specific cytokines pattern [13,14]. IL-27 has a pivotal role in immune regulation and Th cell differentiation and also acts as a mediator between the innate and adaptive immunity [15]. IL-27 is one of the IL-12 family members and mainly secreted by antigen- presenting cells such as macrophage and dendritic cell, it is first described as a pro inflammatory cytokine [16]. It is established that IL-27 has anti- inflammatory role in immune responses by inducing T cells to produce the anti-inflammatory cytokine IL-10 [17].
Our study revealed that, the AA genotype of IL-27 gene was frequent in the patients with SLE when compared with the controls. While, the heterozygous AG genotype was associated with the healthy group, this result denoting that AG genotype may have a protective role against the development of SLE. Also we found a strong links between A allele of IL-27-924A/G and incidence of lupus nephritis (P=0.002) and also with ESR & CRP, indicating that A allele may be have a role in the disease severity. On contrast to our findings, a study on Polish population found that G allele of IL- 27 gene was associated with increased risk of SLE [15]. The SNP -924 A/G is situated in the promoter region of IL-27 gene and hence plays an integral role in the regulation of IL-27 gene expression as it may act as initiator of the transcription [15].
The differences in the genotypes and alleles frequencies between our results and previous researches might be explained by the disease heterogeneity, different ethnic population as well as limited sample size.
IL-18, a pro-inflammatory cytokine, a member of the IL-1 superfamily which performs innate and acquired immunity [18]. Many cells of immunity as macrophages, NK and dendritic cells produce IL-18 and its expression in SLE are directly related with disease activity [19]. IL-18 can enhance lupus-like disease in in some experimental studies on mice [20]. Furthermore, because of the chromosomal location of IL-18 gene at SLE susceptibility locus, this increases the role of IL-18 in genetic susceptibility to SLE [21].
The result of the -607C/A polymorphism of the IL-18 gene in the studied groups revealed that, there were no significant variations either in genotypes frequency (CC, CA and AA) or alleles distribution (C and A) between the two studied groups. These results were in agreement of many European studies [22,23]. On the other hand, Xu et al. [24] reported that GG genotype of IL-18 gene was associated with increased risk of SLE in Chinese population. Also we found a strong links between A allele of IL-18 -607C/A and incidence of lupus nephritis (P=0.001) and also with CRP. The incidence of the IL-18 Alleles and genotypes differ between ethnic groups, and their associations with SLE may be dependent on this distribution of variants as well as the sample size (Figure 3).
Figure 3: Distribution of KOOS Sport/Rec score between baseline
and week 24 in the Undenatured Collagen and Placebo group; †
p<0.05, significant change over time observed in UC-II® group. Note: † p<0.05, significant change over time in Undenatured
Collagen group.
We concluded that -924 AA genotype of IL-27 gene might be a genetic risk factor for SLE susceptibility, while the heterozygous AG genotype may be protective against the disease incidence. In addition, IL-18 gene polymorphism may have no role in SLE disease itself. However, both genes may have a role in lupus nephritis. To investigate the detailed functions of cytokines more specifically, researches involving other types of cytokines on a larger scale of SLE patients are recommended.
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Google Scholar] [PubMed].
Citation: Rizk E, Mohamed A, Badawy A, Mokhtar N, Eid E, Abdelsalam N, et al. (2022) Il-27 and Il-18 Genes Polymorphism in Egyptian Systemic Lupus Erythematosus Patients. J Clin Chem Lab Med. 5:237.
Received: 16-Aug-2022, Manuscript No. JCCLM-22-18872; Editor assigned: 19-Aug-2022, Pre QC No. JCCLM-22-18872 (PQ); Reviewed: 02-Sep-2022, QC No. JCCLM-22-18872; Revised: 09-Sep-2022, Manuscript No. JCCLM-22-18872 (R); Published: 16-Sep-2022 , DOI: 10.35248/JCCLM.22.5.237
Copyright: © 2022 Rizk E, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.