
Immunotherapy: Open Access
Open Access
ISSN: 2471-9552

ISSN: 2471-9552
Case Report - (2022)Volume 8, Issue 3
Introduction: End stage kidney disease (ESKD) and cancer have been identified as risk factors for severe and fatal cases of COVID-19, making vaccination in these patients a priority. Patients suffering from ESKD have a significantly weaker response to common vaccines than general population. However, humoral and cellular immune responses after two doses of RNA-based vaccine BNT162b2 (Pfizer–BioNTech) have been poorly explored in this vulnerable population.
Case presentation: A 69-year-old male patient was followed for ESKD and myeloma treated with Daratumumab. He developed a severe SARS-CoV-2 pneumonia treated with oxygen supplementation and dexamethasone twenty days after two doses of BNT162b2 vaccine. Whole genome sequencing found that the virus belonged to the 20I/501Y.V1 clade. A serology draws eight days after the 2nd vaccine dose showed positive RBD IgG without neutralizing activity against three live viral isolates. A serum specimen sampled thirty days after the onset of SARS-CoV-2 infection showed seroconversion against both RBD and N antigens. This specimen was shown to exhibit a frank neutralizing activity with a higher titer on 20I/501Y.V1 virus than against the reference virus or the 20H/501Y.V2. The QuantiFERON® SARS-CoV-2 (Qiagen) showed a positive specific cellular response although the QuantiFERON monitor displayed a weak cellular response.
Conclusion: Impaired immunity due to renal failure probably explains the severe pneumonia despite vaccination. In fact, it is well-know that dialyzed patients showed bad vaccination response. The fact that the patient develops a neutralizing activity and a cellular response after a third stimulation by infection may suggest to systemically administrating a third supplementary dose of vaccine in ESKD patients as for hepatitis B.
SARS-CoV-2; COVID-19 vaccine; Dialyzed patient; End-stage kidney disease; BNT162b2; Immune response
End Stage Kidney Disease (ESKD) and cancer have been identified as risk factors for severe and fatal cases of COVID-19, making vaccination in these patients a priority [1]. Patients suffering from ESKD have a significantly weaker response to common vaccines than general population [2]. However, humoral and cellular immune responses after two doses of RNA-based vaccine BNT162b2 (Pfizer–BioNTech) have been poorly explored in this vulnerable population. The fact that the patient develops a neutralizing activity and a cellular response after a third stimulation by infection may suggest to systemically administrating a third supplementary dose of vaccine in ESKD patients as for hepatitis B.
A 69-year-old male patient was followed for ESKD and myeloma treated with Daratumumab. He received his first injection of BNT162b2 vaccine on January 2021, 20th and the second one 21 days after, on February 10th. Twenty days after the second dose (March, 1st), the patient was hospitalized at Cannes hospital for fever, asthenia, and polypnea. The patient was tested positive for SARS-CoV-2 on nasopharyngeal sample by a RT-PCR assay performed with the NeuMoDX® (Qiagen) instrument cycle threshold values for targeted genes (N and NSP2) were 13.1 and 14.4, respectively. Whole genome sequencing found that the virus belonged to the 20I/501Y.V1 clade (GISAID number: EPI_ ISL_1312948) with no additional mutation in the spike gene. The blood cell count showed lymphopenia (0.34 G/L, normal range 1-4 G/L) but no inflammatory syndrome (C-Reactive Protein<20 mg/L). The patient developed fever for 8 days and exhibited an oxygen saturation<93%. Computed tomography of the chest showed bilateral ground-glass opacities involving 25 to 50 % of the lung area. The patient was treated with oxygen supplementation (3L/min) and dexamethasone for 8 days with a favorable outcome. A serological test performed before vaccination showed neither receptor binding domain (RBD) (Atellica®, Siemens) nor antigen N specific antibodies (Architect®, Abbott) to SARS-CoV-2. The second serum specimen sampled eight days after the 2nd vaccine dose showed positive RBD IgG [3.92 arbitrary units (AU)/ mL, positive threshold>1 AU/mL] and negative N IgG. A third serum specimen sampled thirty days after the onset of SARSCoV- 2 infection showed a clear seroconversion against both RBD (IgG>150 AU/mL) and N antigens (IgG index of 7.53) (Figure 1). Despite a weak RBD humoral response after vaccination, the patient developed SARS-CoV-2 pneumonia, which raises the question of vaccine efficacy. To better evaluate the humoral protection against SARS-CoV-2, an in vitro virus neutralization assay, using three live viral isolates, was performed on the three serum samples as previously described [3]. Whereas no neutralizing activity was observed on the first two serum specimens (titer<20), including the post-vaccination specimen that exhibited a low level of RBD antibodies, the post-infection specimen was shown to exhibit a frank neutralizing activity with an higher titer against the 20I/501Y.V1 virus (titer of 640) than against the reference virus (titer of 240) or the 20H/501Y.V2 virus (titer of 80) (Figure 1). This observation may explain COVID-19 infection in this patient despite complete vaccination.
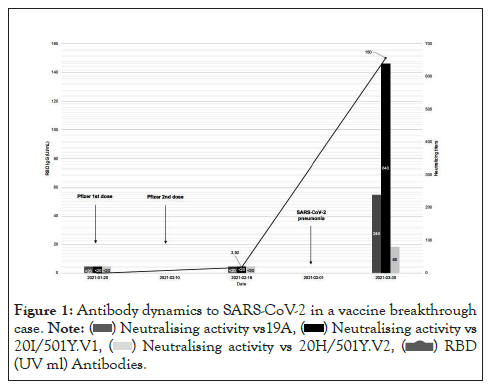
Figure 1: Antibody dynamics to SARS-CoV-2 in a vaccine breakthrough
case.
 (UV ml) Antibodies.
(UV ml) Antibodies.
Impaired humoral responses are well-known in patients on chronic dialysis against vaccines as hepatitis B and influenza [4], which may presume a poor answer against SARS-CoV-2 vaccination. Canas et al. [5] also showed a lack of correlation between neutralizing activity and conventional serology testing on convalescent serum specimens from pediatric dialyzed patients. These observations raise the question of straightening SARS-CoV-2 immunization in dialyzed patients, as recommended with double-dose hepatitis B vaccination in this population [4]. On the sample collected after the infection, interferon-gamma release assay (IGRA) by QuantiFERON Monitor showed a low IFN-y level (3.32 UI/ml) while the QuantiFERON® SARS-CoV-2 (Qiagen) showed a positive specific cellular response. A limit of this case report results is the absence of cellular investigation on the serum specimen taken after the second dose of vaccine to compare cellular immune activity before and after the SARS-CoV-2 pneumonia. Interestingly, the natural infection of this patient with the 20I/501Y.V1 virus boosted both his protective humoral and cellular immune responses to SARS-CoV-2. Another original feature of this case report is the concomitant treatment of this patient with Daratumumab for multiple myeloma. This treatment cannot be made responsible for the poor immune response to SARS-CoV-2 vaccine since Daratumumab was shown to expand both the helper and cytotoxic T-cell repertoires [6].
The chronology of events reported herein needs also to be emphasized; indeed, the SARS-CoV-2 infection occurred within 20 days after the second dose of vaccine, as recently reported by Hacisuleyman et al. in two women infected with SARS-CoV-2 variants despite successful vaccination by BNT162b2 [7]. Unlike these two women our patient presented a severe infection probably explained by impaired humoral immunity due to renal failure. This may suggest to systemically administrating a third dose of vaccine in ESKD patients. Prospective studies are necessary to evaluate the efficiency of this scheme in ESKD patients.
Impaired immunity due to renal failure probably explains the severe pneumonia despite vaccination. The fact that the patient develops a neutralizing activity and a cellular response after a third stimulation by infection may suggest to systemically administrating a third dose of vaccine in ESKD patients. The study concluded that the third dose of vaccine to ESKD patients should provide due to renal failure.
The authors acknowledge the staff of the Cannes hospital laboratory for the realization of SARS-CoV-2 PCR.
The authors declare they have no conflict of interest.
[crossref] [Google Scholar] [PubMed].
[crossref] [Google Scholar] [PubMed].
[crossref] [Google Scholar] [PubMed].
[crossref] [Google Scholar] [PubMed].
[crossref] [Google Scholar] [PubMed].
[crossref] [Google Scholar] [PubMed].
Citation: Manni S, Lotte L, Bal A, Josset L, Lina B, Trabaud MA, et al (2022) Immunity to SARS-CoV-2 in a Dialyzed Patient who Developed COVID-19 Twenty Days After the Second Dose of BNT162b2 Vaccine: A Case Report. Immunotherapy (Los Angel). 08:192.
Received: 02-May-2022, Manuscript No. IMT-22-16467; Editor assigned: 04-May-2022, Pre QC No. IMT-22-16467 (PQ); Reviewed: 19-May-2022, QC No. IMT-22-16467; Revised: 26-May-2022, Manuscript No. IMT-22-16467 (R); Published: 06-Jun-2022 , DOI: 10.35248/2471-9552.22.08.192
Copyright: © 2022 Manni S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.