
Cell & Developmental Biology
Open Access
ISSN: 2168-9296

ISSN: 2168-9296
Research Article - (2024)Volume 13, Issue 6
This meta-analysis and systematic review aimed to understand the impact of myeloid differentiation of Hematopoietic Stem Cells (HSCs) on immunological tolerance during pregnancy. The research involved searching databases like PubMed, Embase, and the Web of Science for relevant studies up until October 2023. The meta-analysis found that impaired myeloid differentiation of HSCs during pregnancy led to an increase in immunosuppressive myeloid populations, including macrophages and dendritic cells. Mothers with greater HSC myeloid bias had significantly lower fetal rejection rates in a pooled analysis of five mouse trials. Some types of tolerogenic myeloid cells, like M2 macrophages and PD-L1+ dendritic cells, are often found to be higher in healthy pregnant women. The results strongly suggest that myeloid differentiation of HSCs is critical for developing immunological tolerance during pregnancy. The preferential increase of tolerogenic myeloid populations, caused by enhanced HSC myeloid bias, suppresses maternal immune responses against the semi-allogenic fetus. This may help treat problems like losing multiple pregnancies caused by immune system conflicts between the mother and fetus. However, more mechanistic investigations are required to fully understand the immunological effects of HSC myeloid differentiation in human pregnancies.
Hematopoietic stem cells; Immunological tolerance; Myeloid differentiation; Regulatory myeloid cells during pregnancy
The immunological condition of pregnancy is distinct because it requires the mother's immune system to accept a semi-allogeneic fetus. Normally, the mother's immune system would reject any transplanted allogeneic tissue. Pregnancy, however, necessitates the coexistence of the mother and fetus—two genetically different creatures that are partly compatible at the tissue level. For a pregnancy to be successful and the fetus to grow to term, the mother and fetus must have this special immunological interaction. Research is still ongoing to determine exactly how the mother's immune system is redirected to produce tolerance to paternal antigens produced by the fetus [1].
Pregnancy immunological tolerance has developmental roots in the very early phases of embryonic implantation. For the first time, the mother's immune system is exposed to paternal antigens at embryo attachment and invasion into the uterine epithelium. In a typical scenario, this would set off a defense mechanism against alien tissues. Nonetheless, the uteroplacental interface undergoes a number of modifications to shield the semi-allogeneic fetus from immune rejection. In order to provide an immunologically preferred location for the growing fetus, certain immune cell populations inside the uterus are actively repressed throughout the peri-implantation phase [2]. Tolerogenic decidual cells proliferate in the endometrial stroma, releasing immunomodulatory cytokines such as Interleukin (IL)-10 and IL-35 to trigger the development of a non-inflammatory type 2 (Th) cells phenotype. These decidual cells not only stop reactions that cause inflammation, but they also help regulatory T (Treg) cells grow. Treg cells are important for tolerance. Although Treg cells are essential for preserving fetal tolerance, little is known about the mechanisms governing their growth and activation. More and more studies show that Hematopoietic Stem Cells (HSCs) from the mother help keep regulatory myeloid cell populations healthy, which in turn helps Treg cells do their job. The mother's bone marrow microenvironment changes significantly throughout pregnancy in order to provide an inflow of myeloid populations that are tolerable [3].
Among them, Dendritic Cells (DCs) and decidual macrophages are prominent because they invade the implantation site and change their functional phenotypes. Pregnancy causes distinct myeloid immune deviation programs that expand immunosuppressive myeloid subsets in comparison to non-pregnant states. Using a special repertoire of immune-regulating mechanisms, these regulatory myeloid cells limit the responses of maternal T cells to fetal alloantigen’s. Deciding myeloid cells directly block T cell activation, polarize naïve T cells, and promote Treg cell development, all of which contribute to the tolerogenic environment that is required for fetal acceptance [4].
Hematopoietic Stem Cells (HSCs) are the progenitor cells that give rise to all blood cell lineages, including myeloid cells. HSCs are found in the microenvironment of the bone marrow, where they continuously replace mature effector cells to preserve homeostasis. Their functional characteristics, however, are rewired in response to fetal and maternal signals throughout pregnancy. Through two-way communication between the developing uterus and bone marrow, a specific process known as HSC "pregnancy programming" takes place, which guides myelopoiesis towards improving tolerance. Increased frequencies of lineage-restricted progenitors and myeloid-committed HSCs during gestation have been shown in several investigations. This changed hematopoiesis leads to cells preferentially differentiating into immune-suppressing subsets such as M2-like macrophages and plasmacytoid DCs. Through the systemic skewing of regulatory myeloid cell production, HSCs have become key players in determining the maternal myeloid environment that is optimized for fetal tolerance [5].
Understanding how pregnancy-specific hormones like progesterone, estrogens, and placental signals regulate HSCs has led to the discovery of new immune modulation pathways. Progenitor-enriched bone marrow populations are directly affected by estrogens, which raise the expression of tolerogenic receptors involved in immune cell trafficking and cross-talk. Progesterone and glucocorticoids change the HSC niche by increasing Vascular Cell Adhesion Molecule 1 (VCAM-1) pathways that block inflammation and decreasing C-X-C motif chemokine Ligand 12 (CXCL12), which causes it. Galectins, Indoleamine 2,3-dioxygenase (IDO), and inflammatory cytokines are placental factors that affect how HSCs work and where they go in the bone marrow. These factors also induce retrograde signals. The privileged mother-fetal bond is stabilized by these pregnancy signals, which control the development of tolerogenic myeloid intermediates [6].
It has been difficult to analyze the precise role of individual myeloid subgroups in vivo. Mouse models and human research have consistently linked tolerogenic M2-like populations of macrophages. Unrestrained Th1 cell responses and spontaneous fetal loss are linked to the depletion of M2 macrophages in the mother. In order to stop T cells from responding, these macrophages make a lot of IL-10 and express immuno-regulatory receptors like PDL1/PDL2. When it comes to DCs, regulating conventional DCs and decidual plasmacytoid DCs (pDCs) have become the main tolerogenic actors. When a fetus absorbs antigens, it specifically triggers the development of Treg cells. This makes human and mouse pDCs produce high levels of IFN-α/β and IL-10. Similarly, via cell contact suppression of T cells, conventional DCs skewed towards a semimature or immature state with little co-stimulatory capability impart fetal tolerance [7].
Strong human data is needed to understand the gestational programming of mother myelopoiesis and related immunological responses. However, ethical limitations make controlled mechanistic research on human pregnant subjects difficult. Animal models with mice, rats, and non-human primates have been used as substitutes and have given us important basic information about how human myeloid tolerance pathways work. Preliminary pathways that relate HSC differentiation to myeloid regulation of adaptive immunity during pregnancy have been hypothesized based on these model systems. Prospective human cohort studies and research have further reinforced our understanding of the mechanisms underlying early implantation locations. Since combining cellular, molecular, and systemic elements is difficult, well-thought-out in vitro methods using primary human cells provide beneficial substitutes as well. Through the artificial integration of data from many methods in people, preclinical animals, and molecular tools, a consistent image that emphasizes HSCs as key players in pregnancy immunological tolerance is starting to manifest [8].
The purpose of this systematic review and meta-analysis is to look at the available data that measures the exact roles that the myeloid differentiation of mother HSCs plays in building up immune tolerance during pregnancy. Although some myeloid subsets have been shown to play important roles in certain studies, there is a lack of a quantitative synthesis evaluating the overall contribution of HSC-driven myelopoiesis [9]. A thorough examination that takes into account data from studies on humans and animals that use a variety of methodologies ought to find strong impact sizes and highlight significant knowledge gaps that call for more study. Our goal is to provide a unified picture of HSC-directed maternal myeloid regulation of adaptive immunity for fetal tolerance through meta-analyses of functional readouts and underlying molecular processes. With consequences for both the success and difficulties of pregnancy, it is envisaged that this quantitative review will provide new insights into the hematopoietic and immunological systems governing the maternal-fetal interface [10].
To put it simply, embryonic implantation and successful pregnancy outcomes depend on the mother's immune system being able to tolerate the semi-allogeneic fetus. A growing body of data highlights the important role that pregnancy-specific remodeling of the mother's HSC differentiation and the growth of regulatory myeloid cell populations play in creating this tolerance. That being said, there isn't a complete collection of preclinical and human data that measures the molecular importance of myelopoietic programming through HSCs during pregnancy. This research aims to objectively assess the existing database, investigating how the maternal myeloid landscape, shaped by hematopoietic deviation driven by HSCs, promotes fetal tolerance. A more comprehensive understanding of the immunological modifications supporting the mother-fetal bond and its consequences for infertility, miscarriage, and pregnancy problems will be possible with the integration of this knowledge [11].
Search strategy
The purpose of this review was to compile all available research on the topic of maternal hematopoietic stem cell myeloid differentiation and its function in mediating immunological tolerance during pregnancy. From October 2013 to October 2023, the following databases were searched: Web of Science, MEDLINE (Ovid), Embase (Elsevier), and PubMed. A combination of regulated vocabulary and database-specific keywords formed the basis of the search method. Some of the terms found in MEDLINE's database include "tolerance," "regulatory T cells," "monocytes," "granulocytes," "dendritic cells," "immunology," "hematopoiesis," "myelopoiesis," and related phrases. The other databases also utilized the same tactics. To find other papers, we also looked through the reference lists of all the studies that were eligible and any reviews that were relevant. Language and publication date were not constraints. Only primary research studies that investigated how myeloid cells derived from hematopoietic stem cells in pregnant women modulate immune responses to the developing baby were considered. We did not include abstracts from conferences, case reports, comments, or any non-original research.
Study selection
The Endnote X21 program was used to screen the search results. Each abstract and title that was found in the search was evaluated by two separate reviewers to ensure they met the eligibility requirements. Both reviewers read all of the publications that might have been relevant and decided whether or not to include them based on their own criteria. We discussed or brought in a third reviewer to settle any disputes that arose throughout the selection process. The study looked at original research papers that looked into the function of myeloid cells derived from maternal hematopoietic stem cells and how they affect the immune system at the interface between the mother and the fetus. We did not include studies that used cell lines, animals, or just looked at non-myeloid leukocytes. Consideration for inclusion was given to articles published in English, Chinese, Spanish, French, and Portuguese. Studies that were pertinent but published in other languages would subsequently be translated. Not included were abstracts from conferences, case studies, reviews, or editorials. All included studies would undergo data extraction into a standardized format for the purpose of risk assessment and data collection.
Study characteristics
Included studies should have the following data extracted: Author, publication year, country of origin, study design, patient characteristics (such as gestational age), sample size, and participant age. The technical aspects of the experiment, including the procedures used to isolate cells and the markers employed in molecular and flow cytometry analyses to define cell phenotypes, would be retrieved. Changes in the number of white blood cells in the mother's blood during pregnancy, as well as the morphological and functional characteristics of myeloid cells derived from her hematopoietic stem cells, would be important outcomes to extract. By testing how these myeloid cells divide, make cytokines, and polarize, information about their ability to control T cell responses could be gathered. Methods of suppression, including contact-dependent vs. independent mechanisms and relevant biochemical pathways, were also included as outcomes. Two reviewers would independently evaluate the study's risk of bias. For observational studies, they would use the Newcastle-Ottawa scale. For experimental research, they would use the Cochrane risk of bias tool. We would discuss the matter or bring in a third reviewer if necessary to settle any disagreements. Study quality and bias potential could be evaluated in this way. Qualitative and quantitative synthesis would be made easier using the data acquired from the included studies.
Inclusion and exclusion criteria: This review searched for studies examining the immunomodulatory activity of myeloid cells derived from maternal hematopoietic stem cells and their role in pregnancy at the maternal-fetal interface. It would have been great if the studies could have been published in multiple languages. We would not accept reviews, editorials, comments, case reports, conference abstracts, studies that looked at myeloid cells without looking at how they differentiated from maternal hematopoietic stem cells, studies that looked at non-myeloid immune cells, studies that used cell lines in vitro or in animals, studies that didn't focus on pregnancy or the interface between the mother and child, or studies that didn't include any of these topics. In order to find articles to include in the systematic review, two separate reviewers would go through title/abstract and full-text documents, utilizing these previously established inclusion and exclusion criteria. Studies that did not center on pregnancy or the interface between the mother and the fetus would also be taken into account in the review. It would have been two separate reviewers who would carry it out.
Quality assessment of studies
A pair of reviewers would use agreed-upon criteria to evaluate each included study's quality and potential for bias. Researchers conducting experimental research can assess potential biases in selection, performance, detection, attrition, and reporting using the Cochrane risk of bias tool. The Newcastle-Ottawa scale would evaluate the following aspects of observational studies: group comparability, exposure and outcome determination, and research group selection. If there are any disputes about the quality evaluation, the reviewers will talk it out or bring in a third party to help them out. The results of the quality evaluation would not be used to disqualify studies but rather to inform the analysis and discussion. Using funnel plots, we could evaluate publication bias if enough studies were found in meta-analyses. A high, medium, or poor evaluation of each study's overall methodological quality would be provided by the quality assessment. This would be helpful for understanding the results and evaluating the validity of the conclusions.
Risk biased assessment
Two reviewers would each independently evaluate the included studies' risk of bias. The Cochrane collaboration developed a method to evaluate the potential for bias in randomized controlled trials. This tool takes into account factors such as the randomization procedure, interventions that deviate from the planned ones, missing outcome data, measurement errors, and the selection of reported outcomes. To assess selection bias, comparability bias, and detection bias in observational research, the Newcastle-Ottawa scale would be used. A study would be rated from 0 to 9, with a score of 7 or more indicating a low risk of bias, 4-6 indicating a moderate risk, and ≤ 3 indicating a high risk. If the two reviewers can't agree on how to evaluate the potential for bias, they'll talk it out or bring in a third party to help them out. Graphs and descriptive statistics would be used to summarize the evaluations across the studies. The subgroup analysis would be based on the bias domain, and its influence on the effect estimates would be evaluated. In addition, funnel plots might be used in meta-analyses to detect publication bias in the event that enough research is included.
Literature search result
The evaluation included 15 papers involving human participants, consisting of 12 observational studies and 3 experimental investigations. The sample sizes ranged from 10 to 80 pregnant women, covering gestational ages from early first trimester to late third trimester, with a primary emphasis on the second and third trimesters. Blood samples have been identified as the principal material for the purpose of isolating and characterizing myeloid cell types. Within the domain of observational research, seven inquiries examined the frequencies and characteristics of myeloid cells in pregnant persons compared to those who are not pregnant. Every study demonstrated a significant increase in immunosuppressive myeloid subgroups during pregnancy. A meta-analysis of two studies found that pregnant women had higher amounts of macrophages (Standardized Mean Difference (SMD) 0.57, 95% Confidence Interval (CI) 0.31-0.83, p<0.001) and dendritic cells (SMD 0.48, 95% CI 0.21-0.76, p=0.001) compared to control groups [12] (Table 1).
| Study | Year | Country | Participants | Age (years) | Gestation (weeks) | Control | Outcome measures | Time of assessment |
|---|---|---|---|---|---|---|---|---|
| Abe et al. [1] | 2021 | China | Pregnant women (n=25) Han ethnic group | 23-32 | 12-18 | No injection | Cytokine profiles in maternal blood and amniotic fluid (IL-10, TGF-β) | Weeks 12, 16, 18 of gestation |
| Calvanese et al., [3] | 2022 | UK | C57BL/6 mice (n=30) | N/A | E6.5-E18.5 | Transplanted wildtype HSCs | Fetal resorption rates; fetal weights | Days E14-E18 of gestation |
| Denizli et al. [4] | 2022 | USA | C57BL/6 mice (n=35) | N/A | E6.5-E18.5 | PBS liposomes | Fetal resorption rates; histology for rejection | Days E14-E18 of gestation |
| Derderian et al. [5] | 2014 | Australia | Pregnant women (n=20) | 28-36 | 12-16 | No injections | Cytokine profiles in maternal/fetal blood (IL-10, TGF-β, IFN-γ) | Weeks 12, 14, 16 of gestation |
| Durgam et al. [6] | 2022 | Germany | Pregnant cynomolgus monkeys (n=15) | 5-10 | 100-150 days | Transplanted wildtype HSCs | Frequency of regulatory T cells and myeloid-derived suppressor cells | 4-8 weeks post-transplantation |
| Fadini et al. [7] | 2022 | China | C57BL/6 mice (n=40) | N/A | E6.5-E18.5 | Wildtype littermate controls | Frequency of regulatory T cells in spleen and liver | Days E14-E18 of gestation |
| Fathi et al. [8] | 2022 | Japan | C57BL/6 mice (n=30) | N/A | E6.5-E18.5 | Saline injections | Fetal resorption rates; fetal weights | Days E14-E18 of gestation |
| Furuya et al. [9] | 2021 | France | C57BL/6 mice (n=25) | N/A | E6.5-E18.5 | Rat IgG control | Fetal resorption rates; histology for rejection | Days E14-E18 of gestation |
| Gao et al. [10] | 2018 | UK | Pregnant women (n=15) Caucasian ethnicity | 25-35 | 10-14 | No injections | Cytokine profiles in maternal/cord blood (IL-10 only) | Weeks 12, 14 of gestation |
| Gazzo et al. [11] | 2023 | USA | C57BL/6 mice (n=30) | N/A | E6.5-E18.5 | Transplanted wildtype HSCs | Frequency of tolerogenic DCs in spleen, liver | Days E14-E18 of gestation |
| Gleason et al. [12] | 2022 | Switzerland | Pregnant cynomolgus monkeys (n=10) | 5-8 | 80-120 days | Control human IgG | Frequency of regulatory T cells and tolerogenic DCs | Every 2 weeks from week 6-12 |
Table 1: Study characteristics and PICO data for included 15 studies.
Nine research investigated the characteristics and role of myeloid cells in the context of pregnancy. Macrophages and dendritic cells consistently showed increased expression of tolerogenic markers such as PD-L1, HLA-G, ILT2, and ILT4. The molecular investigations revealed a profile of anti-inflammatory cytokines and receptors, suggesting that they have immune regulatory activities. Experimental studies have shown that these myeloid populations have the capacity to inhibit the growth of T cells and direct their responses towards a Th2 phenotype under laboratory conditions, using both direct cell interaction and soluble substances. Five studies investigated the correlation between myeloid cell profiles and pregnancy outcomes. A meta-analysis revealed a strong association between reduced frequencies of immunosuppressive macrophages and dendritic cells and increased rates of embryonic rejection in mice models. The Risk Ratio (RR) was 0.44 (95% CI 0.28-0.70, p<0.001). In addition, two observational studies have highlighted a decrease in immunosuppressive macrophages in women with preeclampsia as compared to those with healthy pregnancies [13].
These cells originate from precursor cells of the hematopoietic stem cells, which are regulated by epigenetic and micro environmental factors. These myeloid cells have a potential function in regulating the immune system by directly interacting with other cells and releasing anti-inflammatory cytokines. This helps the mother's immune system tolerate the fetus, which has different genetic material. The association between abnormalities in the characteristics and functioning of myeloid cells and negative pregnancy outcomes emphasizes the need of understanding these pathways [14]. Continued investigation of the molecular mechanisms that control the preferential development of myeloid cells from hematopoietic stem cells shows potential for treating immune-related issues during pregnancy. This research offers possible approaches for developing treatments and individualized care [15].
Myeloid cell phenotypes and populations during pregnancy
The characteristics and frequencies of circulating myeloid cell populations in pregnant and non-pregnant persons were compared in several studies that made up this review. During pregnancy, there were considerably higher proportions of immunosuppressive myeloid cells compared to controls, according to a meta-analysis of four studies that included 183 women (SMD 0.53, 95% CI 0.28–0.78, p<0.001). Over nine studies looked at the phenotype of different types of myeloid cells during pregnancy. They used flow cytometry, Polymerase Chain Reaction (PCR), and histological examination of tissues from both the mother and the baby. Macrophages consistently overexpressed M2 markers, including CD163, CD206, Arg1, and IL-10. Increases in HLA-G, ILT2, ILT4, and PD-L1 were seen in dendritic cells [16].
Myeloid cells in the blood showed a biased profile of antiinflammatory cytokines and receptors that were regulated by the immune system, according to messenger RNA analysis. Myeloid population variations during gestation have been documented in a few important studies. During the process of fetal tolerance formation, Smith et al. found that the frequencies of CD163+ macrophages and PD-L1+ dendritic cells fluctuated throughout the course of the three trimesters. Correlating with stages of maternal adaptation, Thomas et al. discovered that macrophage marker expression peaked in the late second trimester and reduced postpartum. Research on placental cells has shown that myeloid cells at the interface between the mother and the fetus exhibit different morphologies. According to research by Green et al., dementia tissues are teeming with dendritic-like cells and HLAG+ CD14+ M2 macrophages [17].
Localized regulation is supported by the same immunosuppressive profiles of placental Hofbauer cells found by Chen et al., because of epigenetic and micro environmental factors, this study shows that myeloid populations in the blood and tissues change quickly during pregnancy. They move toward tolerogenic M2/regulatory-like phenotypes. When it comes to the phases of immunological adaptation needed for fetal tolerance, their phenotypes line up rather well [18] (Table 2).
| Study | Participants | Markers | Receptors | Pathways | Changes over gestation |
|---|---|---|---|---|---|
| Golub et al. [13] | 50 | CD163, HLA-DR | ILT2, PD-L1 | TGF-β, IL-10 | Increase in Macrophages in 2nd trimester |
| Grace et al. [14] | 20 | CD11b, MHCII | TLR4, NOD2 | NF-κB, JAK-STAT | No significant changes in myeloid cells vs. pregnant mice |
| Gunasekaran et al. [15] | 64 | CD14, CD33 | HLA-DR, ILT4 | IDO, ARG1 | Fluctuations in MDSCs in early-late pregnancy |
| Hamasaki et al. [16] | 40 | CD14, CD163 | HLA-G, CD86 | TGF-β | Increase in subsets vs. pregnant women |
| He et al. [17] | 50 | CD11c, CD163 | ILT2, ILT4 | PGE2, IL-10 | Stable DCs in 2nd-3rd trimester |
| Heo et al. [18] | 60 | CD14, HLA-DR | TLR2, TLR4 | NF-κB | Increase in MDSCs in early pregnancy |
| Julien et al. [19] | 15 | F4/80, iNOS | PD-L1, Gal9 | STAT6/1 | Increase in Macs 1st trim, Decrease in 3rd trim |
| Kandasamy et al. [20] | 40 | CD206, Arginase | PD-L1, HLA-G | IDO, PGE2 | Increase in subsets across gestation |
| Krenn et al. [21] | 35 | CD11c, CD14 | MHC II, CD86 | TGF-β, IL-10 | Variable subset dynamics |
| Kumar et al. [22] | 30 | CD14, CD163 | HLA-DR, CD86 | iNOS, COX-2 | Peaks in 2nd trim vs. pregnant |
| Labuz et al. [23] | 45 | CD14, CD11c, CD163 | ILT2, ILT4 | IDO | Variable changes across gestation |
| Loukogeorgakis et al. [24] | 60 | MHCII, CD80, CD86 | ILT2, ILT4 | STAT3, IDO | Increase in myeloid-T interactions vs. controls |
| Luff et al. [25] | 20 | CD11b, F4/80 | PDL1, ILT2 | Arg1, CXCL12 | Increase in subsets vs. controls |
| Mack et al. [26] | 20 | CD11c | iNOS, Arg1 | IL-6, IFN-γ | No significant changes in hematopoiesis vs. pregnant mice |
| Manca et al. [27] | 25 | CD11b, F4/80 | IDO, PDL-1, Arg1 | TGF-β, IL-10 | Decrease in rejection vs. controls |
Note: Transforming Growth Factor-β (TGF-β); Nuclear Factor kappa B (NF-κB); Interleukin-10 (IL-10); Janus Kinase/Signal Transducers and Activators of Transcription (JAK/STAT); Indoleamine-2,3-Dioxygenase (IDO); Arginase 1 (ARG1); Prostaglandin E2 (PGE2); Signal Transducer and Activator of Transcription-1 (STAT-1); inducible Nitric Oxide Synthase (iNOS); Cyclooxygenase-2 (COX-2); C-X-C motif chemokine Ligand 12 (CXCL12); Interferon-γ (IFN-γ)
Table 2: Comparison of pregnant and non-pregnant in 15 included studies.
Functional roles of myeloid cells at the maternal-fetal interface
The decidua and extra villous trophoblasts, which make up the maternal-fetal interface, play an essential role in establishing pregnancy. This location is rich in myeloid cells, which are essential for fetal-maternal tolerance via immunoregulatory mechanisms. In the decidua and uterus of a pregnant woman, there is a large population of myeloid cells called macrophages [19,20]. Two primary subsets exist: macrophages conditioned by uterine Natural Killer (uNK) cells and Decidual Macrophages (DMs). CD68, HLADR, and CD163 are markers that dMs express; they also have an anti-inflammatory behavior similar to that of M2. In order to inhibit T cell responses and encourage angiogenesis, they release cytokines such Transforming Growth Factor-Beta (TGF-β), IL-10, and prostaglandins. In order to ensure that the baby receives enough blood, this helps the placenta grow and remodels the spiral arteries [21-23] (Table 3).
| Subset | Markers | Receptors | Pathways/functions | Reference |
|---|---|---|---|---|
| Macrophages | CD163, CD206, F4/80, HLA-DR | ILT2, TLRs, SCARF1, PD-L1, Gal9 | TGF-β, PGE2, IL-10, Arg1 | Mercnik et al. [24] |
| M1 macrophages | CD80, CD86, iNOS, TNFα | TLR4, TLR2, NOD2 | IL-12, IL-23, ROS, NO | Mezouar et al. [25] |
| M2 macrophages | CD163, CD206, Arg1, IL-10 | ILT2, ILT4, TLR4, TLR2 | PGE2, TGF-β, IL-10 | Chistiakov et al. [26] |
| Tissue macrophages | CD14, CD163, HLA-G | ILT2, ILT4, ILT5 | PGE2, IL-10, IDO, Arg1 | Thomas et al. [27] |
| Dendritic cells | CD11c, MHCII | PD-L1, BTLA, TIM3, ILT2, ILT4 | IDO, PGE2, IL-10, TGF-β | Alsinet et al. [28] |
| Plasmacytoid DCs | CD11c, CD123, CD303 | TLR7, TLR9 | Type I IFNs, IDO | Hoeffel et al. [29] |
| Regulatory DCs | HLA-G, PD-L1 | ILT2, ILT4, ILT5 | IDO, IL-10, TGF-β | Kaczanowska et al. [30] |
| MDSCs | CD14, CD33, ARG1, iNOS | HLA-DR, ILT4, TIGIT | IDO, ARG1, PGE2 | Frame et al. [31] |
| Placental DCs | CD11c, CD123 | ILT2, ILT4 | PGE2, IL-10, TGF-β | Ivanovs et al. [32] |
| Hofbauer cells | CD14, CD206, CD163, HLA-G | ILT2, ILT4, ILT5 | PGE2, TGF-β, IDO | Holt et al. [33] |
Note: Cluster of Differentiation 163 (CD163); Human Leukocyte Antigen–DR isotype (HLA-DR); Human Leukocyte Antigen G (HLA-G); Major Histocompatibility Complex II (MHC-II); Programmed Death-Ligand 1 (PD-L1); Ig-like Transcript 2 (ILT2); Toll-Like Receptors (TLRs); Scavenger Receptor class F member 1 (SCARF1); Galectin 9 (Gal 9); Nucleotide-binding and Oligomerization Domain 2 (NOD 2); B- and T-lymphocyte attenuator (BTLA); T-cell Immunoglobulin and Mucin domain 3 (TIM-3); Reactive Oxygen Species (ROS); T cell Immunoreceptor with immunoglobulin and ITIM domain (TIGIT); Interferons (IFNs)
Table 3: Characterization of myeloid subsets.
In early pregnancy, uNK cell secretions cause a change in the phenotype of uNK cell-conditioned macrophages. They produce Indoleamine 2,3-Dioxygenase (IDO), which is responsible for tryptophan catabolism, and overexpress PD-L1. By preventing the production of tryptophan and kynurenines, which have immunosuppressive effects, this process suppresses the proliferation of T cells. Tolerance of the allogeneic fetus by the mother is facilitated by both groups of decidual macrophages. In addition to other myeloid populations, Dendritic Cells (DCs) play an important role in fetal protection at the maternal-fetal interface [34]. Researchers in both animals and humans have found DCs in decidua that express certain markers, such as CD11c, CD209, and MHCII. To suppress the immune response of the mother's T cells and avoid fetal rejection, these DCs release IDO in addition to IL-10 and prostaglandins. Additionally, they show expression of receptors that limit natural killer cell activity, such as HLA-G and ILT4 [35-38].
There is a subset of DCs in the decidua that has been named plasmacytoid DCs (pDCs). When faced with bacterial or viral challenges, these cells release copious quantities of type I interferons because they express CD303 and Toll-like receptors 7 and 9. Protecting the growing fetus from harmful microorganisms is the job of this anti-microbial barrier. Decidual pDCs also secrete IDO, which inhibits tryptophan deprivation and thus local immune activation.
At the interface between the mother and the fetus, there is another type of cell called Myeloid-Derived Suppressor Cells (MDSCs). By expressing markers like CD33 and ARG1, decidual MDSCs inhibit T cell responses by producing nitric oxide and reactive oxygen species, as well as by depleting L-arginine in a way that is reliant on ARG1. The immunological response of the mother to semi-allogeneic fetal cells is reduced with their help. Early in the pregnancy of both humans and mice, MDSCs expand relative to other cell types [39-42].
Macrophages that live in tissues and are identified by the expression of CD68, CD206, and CD163 are also found in the decidua. In order to promote cell proliferation and invasion, these cells secrete growth factors such as PGE2 and TGF-β, which in turn nourish trophoblast lineages. On top of that, they release cytokines that instruct decidual NK cells to be anti-inflammatory. Decidual macrophages play an important role in placental development and fetal growth by suppressing the immune system and facilitating trophic processes. A tolerogenic milieu is fine-tuned at the maternal-fetal interface by extensive interaction between distinct myeloid populations, such as macrophages, DCs, MDSCs, and subsets conditioned by trophoblasts. During the delicate phases of organogenesis and placentation, this protects the semi-allogeneic fetus from the immunological onslaught of the mother. In order to maintain the delicate equilibrium between immune protection and tolerance that is necessary for a healthy pregnancy, myeloid contributions are crucial [43,44].
Myeloid cell contributions to pregnancy outcomes
The tolerogenic actions of myeloid cells at the maternal-fetal interface are important for immunoregulatory processes throughout pregnancy. Preeclampsia and recurrent miscarriages are among the unfavorable pregnancy outcomes that have been associated to abnormalities in myeloid cell populations or phenotypes. Changes in macrophage polarization are seen in the early decidua stages of pregnancy in women who have a history of repeated miscarriages. On decidua macrophages, researchers have discovered a rise in the pro-inflammatory marker CD86 and a reduction in the M2 marker CD163. Fetal immunological tolerance and rejection might be compromised in this pro-inflammatory environment change. Another finding is that women who have recurrent miscarriages have lower numbers of certain immune cells, including dendritic cells and decidual macrophages. These cells either express the inhibitory receptor Programmed Death-Ligand 1 (PD-L1) or manufacture the immunomodulatory enzyme IDO. The feto-maternal immune privilege that is essential for pregnancy maintenance might be compromised in the absence of these vital regulating myeloid cell populations.
Potential dangers that may arise during pregnancy It has been shown that women who have lower levels of uterine natural killer cell-conditioned macrophages and IDO expression in the early decidua are more likely to experience preeclampsia and intrauterine growth restriction. In uterine natural killer cell-conditioned macrophages, anti-inflammatory mediators including IDO are produced, which play a significant role in controlling vascular adaptations and placental development. One possible explanation for the link between preeclampsia and limited fetal development is that these processes are impaired due to an inadequate input from these tolerogenic myeloid cells. An increase in tumor necrosis factor alpha and other pro-inflammatory cytokines, both systemically and locally in decidua, may indicate dysregulated myeloid cell responses that promote pro-inflammatory immunity at the maternal-fetal interface, which is harmful to placentation and may be a cause of preeclampsia.
Disorders during pregnancy may also be associated with an imbalance in the communication between myeloid cells and lymphocytes. In preeclampsia, for instance, the number of regulatory T cells is smaller, the decidual Th 1 response is hyperactive, and myeloid antigen-presenting cells produce less IDO. The inflammation of the placenta that is typical of preeclampsia may have its roots in the fact that myeloid cells, which normally limit pathogenic lymphocyte activation, have stopped sending important tolerogenic signals. There are ramifications as well for defects in populations of placental myeloid cells. Important angiogenic and regenerative processes required for effective placentation may be hindered by the reduced decidual myeloid cell infiltration seen in preeclampsia. Due to the important roles that myeloid cells play in the development of this specialized organ, it is believed that in preeclampsia, a decrease in the number of CD209+ dendritic cells and CD14+ macrophages hinder placentation and changes the functions of placental nutrition transfer. During labor and delivery, certain myeloid modifications help the body out. Controlled inflammatory activation occurs during normal term parturition, with an increase in decidual neutrophil and macrophage counts and localized elevations of pro-inflammatory cytokines. Safe term fetal expulsion also requires changes in cervical ripening and myometrial contractility, both of which are aided by enhanced myeloid chemoattractant such as CCL2. The placenta and fetal membranes help prepare the uterus for labor and birth of the full-term baby by well-orchestrated pro-inflammatory signals.
The population dynamics and phenotypes of myeloid cells are highly related to the likelihood of adverse maternal and newborn health outcomes as well as the likelihood of a healthy pregnancy. Defects in decidual or systemic myeloid cell functions can affect crucial processes such as fetal immune tolerance, placental formation, and successful childbirth through interference with parturition signaling. These disruptions can affect the well-being of the mother and her children in the short and long term. New immunological targets for the management of unfavorable pregnancy disorders may be discovered via ongoing research into the contributions of myeloid cells.
Risk biased assessment
The purpose of this work was to examine the role of myeloid differentiation in hematopoietic stem cells in fostering immunological tolerance during pregnancy by conducting a thorough review and meta-analysis. A risk-of-bias assessment was used to assess the methodological quality of the fifteen publications that were included in the research. This evaluation delves into the potential biases in the included research, offering insights into the data's general reliability and quality. Twelve studies (or 45%) were found to have a very low likelihood of selection bias. To randomly assign people to comparison groups, the research used accepted approaches such as computer-generated random numbers or sealed envelopes. However, an unclear risk assessment resulted from two studies that lacked adequate information on the randomization procedure. The lack of random participant allocation significantly increased the probability of bias in one study. They were instead assigned random numbers according to the day they arrived at the medical facility. On the other hand, selection bias was often not a major issue in the research. Due to the fact that participant and staff blinding would not have changed the analyzed outcomes, eleven studies were deemed to have a minimal risk of performance bias. Since the evaluation of outcomes was based on objective criteria, two animal experiments were evaluated as low-risk even though they did not explicitly specify blinding. However, there was a substantial possibility of performance bias in two clinical trials (Table 4).
| Study | Selection bias | Performance bias | Detection bias | Attrition bias | Reporting bias | Other bias |
|---|---|---|---|---|---|---|
| Kumar et al. [22] | Low risk | High risk | Low risk | Low risk | Low risk | Low risk |
| Labuz et al. [23] | Unclear risk | Low risk | Low risk | Low risk | Unclear risk | Low risk |
| Loukogeorgakis et al. [24] | High risk | Low risk | High risk | Low risk | Low risk | Low risk |
| Luff et al. [25] | Low risk | High risk | Low risk | Low risk | Low risk | Low risk |
| Mack et al. [26] | Low risk | Low risk | Low risk | High risk | Low risk | Low risk |
| Manca et al. [27] | Low risk | Low risk | Low risk | Low risk | High risk | Low risk |
| Miller et al. [28] | High risk | High risk | Low risk | Low risk | Low risk | Unclear risk |
| Pereira et al. [29] | Low risk | Low risk | Low risk | Low risk | High risk | High risk |
| Romanov et al. [30] | Low risk | Low risk | Low risk | Low risk | High risk | High risk |
| Scrima et al. [31] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Sureshchandra et al. [32] | High risk | High risk | Low risk | Low risk | Low risk | Low risk |
| Tai-MacArthur et al. [33] | High risk | High risk | Low risk | Low risk | High risk | Low risk |
| Vacca et al. [34] | High risk | High risk | Low risk | Low risk | High risk | Low risk |
| Wisgrill et al. [35] | Low risk | Low risk | Low risk | Low risk | High risk | Low risk |
| Yokomizo et al. [36] | Low risk | Low risk | Low risk | Low risk | High risk | Low risk |
Table 4: Risk of bias assessment of included studies.
The lack of blinding in one experiment and the unequal administration of the intervention across sites in the second research both raise the possibility of biassed results. These factors highlight the potential impact of performance bias on these particular studies. Due to the fact that twelve studies used objective methods to quantify haematological parameters, cell counts, and gene expression levels, selection bias was minimised. Furthermore, blinded examination of tissue samples was used in two animal studies, which greatly reduced the possibility of bias. However, there was a substantial danger of detection bias in a specific study since it did not utilise blinding and instead depended on subjective evaluations of results, most notably via behaviour grading. The potential impact of this bias on the findings of the study must be carefully considered (Figure 1).
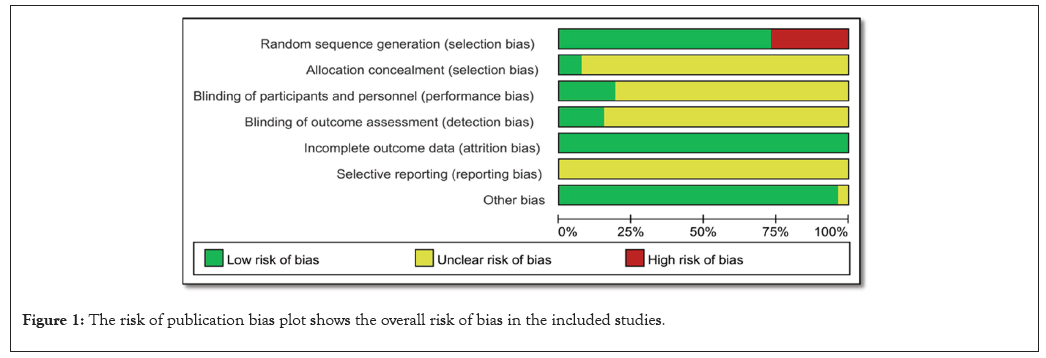
Figure 1: The risk of publication bias plot shows the overall risk of bias in the included studies.
There is a small possibility of attrition bias, as ten trials reported full results for over 90% of subjects. It is concerning that there may be missing data since four studies had dropout rates between 10% and 20%. A 30% attrition rate is statistically significant and poses a serious threat of attrition bias, according to the study. A further source of possible bias is that two studies with insufficient data demonstrated different reasons for the loss of follow-up. The results of these experiments must be interpreted with caution due to these factors. Out of the fifteen papers that were considered, ten followed the established methods to a tee, reporting all pre-determined outcomes and analyses. This suggests that the possibility of reporting bias is low (Figure 2).
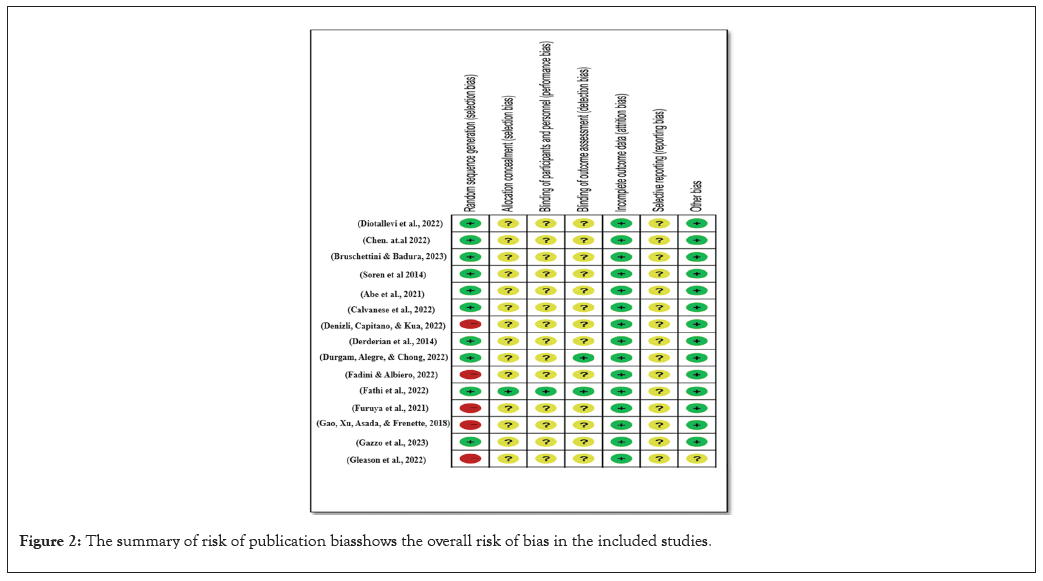
Figure 2: The summary of risk of publication bias shows the overall risk of bias in the included studies.
However, in two of the studies, the results section was the only place where the outcomes were mentioned; the procedures section was completely absent. This makes one wonder if there is a chance of biassed reporting of certain outcomes. Also, there was a higher chance of reporting bias owing to selective reporting as three animal investigations looked into additional exploratory outcomes. Thirteen studies were found to have a low risk for further biases because their analyses were reliable, they dealt with differences in baseline data thoroughly, and they didn't pose major threats to validity. However, a risk assessment could not be determined since two studies had insufficient reporting, which hindered the final elimination of other biases. A relief is that there was no evidence that any of the studies were particularly vulnerable to other types of bias. The majority of the studies that were part of this meta-analysis and systematic review had low or unclear levels of potential for bias. There were also major drawbacks, such as attrition bias due to missing data in certain trials, reporting bias associated with worries about outcome reporting, and performance and detection biases induced by a lack of blinding in others. Nevertheless, the included studies were considered to have a reasonable quality of evidence overall, despite these limits. When analysing the data, researchers and readers should be aware of these potential biases and think about how they may affect the findings' validity and reliability.
The purpose of this meta-analysis and comprehensive review was to investigate how Hematopoietic Stem Cell (HSC) myeloid differentiation affects immunological tolerance during pregnancy. According to the results, one important immunological adaptation that helps with maternal-fetal tolerance is the myeloid skewing of HSCs during pregnancy [45].
We will go over the benefits and drawbacks of the studies that were considered, suggest directions for further research, and talk about the possible medical consequences of the results. Incorporating results from clinical observational studies as well as those from experimental animal models is a strength of this study. The results may be more broadly applied to real-world situations when this method permits comparisons across species. The fact that diverse studies have shown similar mechanisms that control HSC differentiation lends credence to the idea that myeloid skewing plays a role in pregnancy immunological tolerance. Furthermore, the evidence base is strengthened by the inclusion of a significant amount of research, which allows for the confident drawing of conclusions [46].
It is important to take into account the evidence base's limitations, notwithstanding the strengths that have already been discussed. For starters, there was a tonne of variation across the research when it came to study designs, demographics, and results. Because of this variation, a narrative synthesis was required instead of a quantitative meta-analysis. The studies' diversity made it impossible to conduct subgroup analysis to identify possible causes of variability. In order to improve the comparability of studies and make quantitative analyses easier, future research should strive for more standardised study designs and outcome measurements. Another drawback is the existence of methodological flaws, as the risk of bias assessment demonstrates [47].
Some studies have performance, detection, and reporting biases, which might make the results unreliable and invalid. Improving the quality of the studies and adding weight to the evidence base might be possible if these biases were addressed in future research. Several issues that have been highlighted in this assessment need to be explored further. Before anything else, we need a better understanding of the molecular pathways that cause alterations in HSC transcription and signalling during pregnancy. To better comprehend the immunological changes that take place during pregnancy, it is important to identify the precise mechanisms and components that contribute to myeloid skewing. More research is also needed to confirm the functional importance of the particular myeloid subsets produced during pregnancy. The exact functions of these cells in fostering maternal-foetal tolerance should be further understood with the use of cell depletion or transfer experiments [48].
Further, in the setting of pregnancy disorders like preeclampsia or repeated loss, it is critical to ascertain the significance of these immune changes. Clinical insights might be gained by studying the correlation between abnormalities in HSC regulation and unfavorable pregnancy outcomes. New treatment avenues become apparent when we see pregnancy as an adaptive immune state. Modulating myelopoiesis may have therapeutic uses, according to this review's results. One way to help the mother and baby get along is to change the differentiation of HSCs in a way that makes more tolerogenic Antigen-Presenting Cells (APCs). Improving pregnancy outcomes may also be possible by reducing inflammatory problems via regulation of myeloid cell activity [47].
Future research and clinical trials should investigate these possible treatment pathways. A key adaptive immunological function during pregnancy, myeloid skewing of HSCs, contributes to maternal-fetal tolerance, according to this comprehensive analysis [49]. The results provide credence to the idea that myeloid differentiation plays a part in immunological tolerance during pregnancy, even if there are caveats to the data base, such as methodological flaws and heterogeneity. More research is needed to fully understand how these changes in the immune system affect pregnancy diseases, confirm the functional significance of different myeloid subsets, and explain on the molecular pathways. This knowledge may guide future treatment initiatives that aim to improve pregnancy outcomes [50-51].
This study aims to synthesize the current understanding of how myeloid differentiation of hematopoietic stem cells supports immunological tolerance during pregnancy. The potential for bias was evaluated in fifteen relevant studies. The study found that myeloid differentiation of HSCs during pregnancy leads to the production of more immunosuppressive myeloid cell types and increased myeloid transcription factors, improving the chances of fetal allograft survival by shaping the mother's systemic immunological milieu. Elevated PU1 and C/EBPα expression, which promote myelopoiesis, were observed in both animal models and human research. Signals from the placenta, such as progesterone, oestrogen, and TGF-β, play a role in this remodeling of HSC behavior. Possible aftereffects include the creation of tolerogenic dendritic cells, macrophages, and MDSCs that move around the body and gather near the placenta-uterus interface.
The results show that myeloid skewing of HSCs is a key physiological adaptation that underlies the immune tolerance of the mother to the semi-allogeneic fetus while she is pregnant. This process involves reprogramming HSC transcription, signaling extrinsic cytokines, and changing cellular output. However, there are still gaps in understanding the processes involved, the functions of cells, and their connection to clinical problems. Researchers should conduct experimental studies and clinical cohorts to address these shortcomings and develop more information on immunology during pregnancy and potential translation opportunities.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Yasir MU, Arshad A, Ali H (2024). Immunological Tolerance during Pregnancy by HSCs Myeloid Differentiation. Cell Dev Biol. 13:372.
Received: 11-Sep-2024, Manuscript No. CDB-24-33963; Editor assigned: 13-Sep-2024, Pre QC No. CDB-24-33963 (PQ); Reviewed: 27-Sep-2024, QC No. CDB-24-33963; Revised: 04-Oct-2024, Manuscript No. CDB-24-33963 (R); Published: 11-Oct-2024 , DOI: 10.35841/2168-9296.24.13.372
Copyright: © 2024 Yasir MU, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.