
Immunotherapy: Open Access
Open Access
ISSN: 2471-9552

ISSN: 2471-9552
Review Article - (2024)Volume 10, Issue 1
Liver is a crucial organ involved in many immunological processes, including clearance of pathogens, tolerance to self-antigens and regulation of inflammation. It receives direct blood supply via the gut portal vein, which contains dietary antigens and metabolites from resident microbiome. However, these processes can be disrupted by various factors leading to liver diseases. In this review, we have summarized the recent developments in fibrosis, cirrhosis, autoimmune diseases and infectious diseases. Fibrosis and cirrhosis are characterized by the accumulation of scar tissue in the liver resulting from chronic inflammation and injury. The immune response to this injury involves the activation of hepatic stellate cells, immune cells and cytokines, leading to inflammatory processes and fibrosis. Autoimmune liver diseases, such as autoimmune hepatitis and primary biliary cirrhosis, result from dysregulated immune responses directed against liver cells. Infectious diseases such as hepatitis A, B, C, D and E also lead to chronic inflammation of liver diseases. Understanding the immunology of the liver is critical for the development of effective therapies. Current treatments focus on immune modulation and anti-inflammatory therapies. The advent of novel therapies such as cell and gene therapies, have unleashed the potential of targeting novel pathways in the pathogenesis of the liver. Future therapies may target specific immune pathways in the immune pathogenesis of the liver pathology.
Liver; Fibrosis; Cirrhosis; Autoimmunity; Viral hepatitis; Inflammation
NAFLD: Non-Alcoholic Fatty Liver Disease; ALD: Alcoholic Liver Disease; AILDs: Autoimmune Liver Diseases; HSCs: Hepatic Stellate Cells; LSECs: Liver Sinusoidal Endothelial Cells; LPCs: Liver Progenitor Cells; NASH: Non-Alcoholic Steatohepatitis; TGF-β: Transforming Growth Factor-Beta; PDGF: Platelet-Derived Growth Factor; DAMPs: Damage-Associated Molecular Patterns; HV: Viral Hepatitis; HCC: Hepatocellular Carcinoma; MMPs: Matrix Metalloproteinases; AIH: Autoimmune Hepatitis; PBC: Primary Biliary Cholangitis; PSC: Primary Sclerosing Cholangitis; APCs: Antigen Presenting Cells; AMAs: Anti-Mitochondrial Antibodies; AE2: Anion Exchanger2; BEC: Biliary Epithelial Cell; IBD: Inflammatory Bowel Disease; UDCA: Ursodeoxycholic Acid; WHO: World Health Organization; HAV: Hepatitis A Virus; HEV: Hepatitis E Virus; HBV: Hepatitis B Virus; HCV: Hepatitis C Virus; HDV: Hepatitis D Virus; PRRs: Pathogen Recognition Receptors; IFN: Interferon; PBMCs: Peripheral Blood Mononuclear Cells; TLRs: Toll-Like Receptors; STING: Stimulator of Interferon Gene; RIG-I: Retinoic Acid-Inducible Gene-I
Liver diseases remain one of the top ten causes of morbidity and mortality. These diseases are responsible for approximately 2 million deaths per year worldwide, 1 million due to complications of cirrhosis and another million due to viral hepatitis and hepatocellular carcinoma [1]. Endogenous liver diseases are classified into Non-Alcoholic Fatty Liver Disease (NAFLD) and Alcohol-associated Liver Disease (ALD), both of which contribute to cirrhosis and liver cancer. Infection of hepatocytes, particularly by hepatitis viruses, also contributes to significant acute and chronic liver pathologies. Epidemiological studies over the past decade have indicated an increase in Autoimmune Liver Diseases (AILDs) [2-4]. The role of the immune response in pathogenesis liver diseases requires careful evaluation. In this review, we have focused on non-alcohol-related liver diseases such as liver cirrhosis, fibrosis, autoimmune liver diseases and viral hepatitis. We have also commented on different immune pathways related to liver and recently developed therapies against liver diseases. We have excluded alcohol-induced liver disease and liver cancer; these are reviewed elsewhere [5,6].
Structure of liver
Liver is a large internal organ weighing about 1200-1500 g. It is located adjacent to the stomach. It receives blood from the hepatic artery and drains blood into the hepatic portal vein. It is responsible for metabolism, detoxification, bile production and immune surveillance. Liver is roughly triangular, consisting of two major lobes: larger right lobe and smaller right lobe. These lobes are separated by a falciform ligament. It also consists of two even smaller lobes: caudate lobe and quadrate lobe. All these liver lobes are further divided into eight segments and multiple lobules (Figures 1 and 2). Liver is composed of a diverse array of cells among which hepatocytes are the predominant ones [7,8].
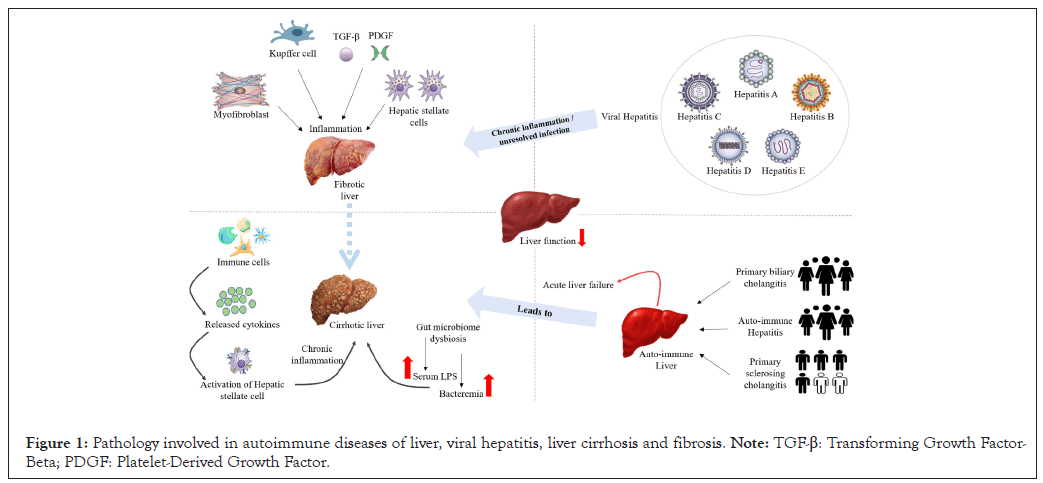
Figure 1: Pathology involved in autoimmune diseases of liver, viral hepatitis, liver cirrhosis and fibrosis. Note: TGF-β: Transforming Growth Factor- Beta; PDGF: Platelet-Derived Growth Factor.
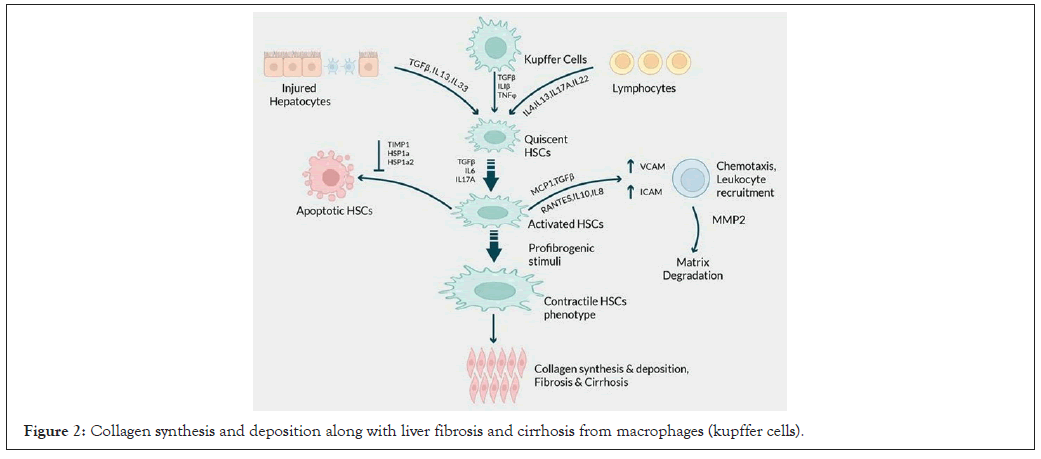
Figure 2: Collagen synthesis and deposition along with liver fibrosis and cirrhosis from macrophages (kupffer cells).
Hepatocytes are epithelial cells that help metabolism by secretion of bile components and detoxification process. These cells are normally in a quiescent stage in a healthy liver. Kupffer cells are specialized macrophages that help in ion homeostasis, phagocytosis and wound healing. Hepatic Stellate Cells (HSCs), also known as ito cells, are mesenchymal cells that help in fibro-genesis as well as uptake and regulation of retinol (vitamin A) [9]. These cells are known to have stem cell-like properties and can further develop into endothelial cells or hepatocytes. Liver Sinusoidal Endothelial Cells (LSECs) form the endothelium, and their main function is effective endocytosis by which they take up required substances from blood stream [10]. Hepatic Natural Killer (NK) cells are also known as pit cells due to their presence in the form of a number of small, peculiar granules. The role in naïve, activated and exhausted states of immune cell types of results in diverse outcomes upon interactions with hepatic stellate cells [11]. The complex interactions of these cell types in the liver play critical roles in maintaining liver immune homeostasis through regulation of inflammatory responses and immune tolerance during pathogenesis of liver diseases.
Liver regeneration
Out of all the solid organs in the body, the inherent ability of liver to regenerate has been extensively studied [12]. Post liver injury, the primary mode of liver regeneration is driven by the proliferation of existing hepatocytes, whereas the secondary mode involves the dedifferentiation of hepatocytes into Liver Progenitor Cells (LPCs or oval cells) and their further differentiation into hepatocytes [13]. LPCs are not found in healthy liver but are present in between the bile duct and hepatic cells in the case of liver damage [13]. Recent studies on molecular mechanisms involved in liver regeneration have shown epigenetic alterations in hepatocytes. These events are driven by chromatin alterations involving hetero-chromatinization and transcriptional repression [14]. Liver regeneration reverses these patterns and rejuvenates multiple molecular and physiological aspects of cellular metabolism. These studies have implications to new treatment regimens for treating liver diseases.
Regulation of inflammation through cytokines in the liver
A healthy liver produces a variety of cytokines required for immune regulation and during inflammation [15-17]. Pro-inflammatory interleukins such as Interleukin (IL)-6, IL-8 and IL-10 are involved in response to infection and recruitment and activation of immune cells such as neutrophils to the sites of inflammation. They also help in the prevention of autoimmune diseases [18]. IL-1-b is involved in the upregulation of immune responses and inflammation, whereas IL-1RA suppresses inflammatory response [19]. Tumor Necrosis Factor-α (TNF-α) is involved in cell death, whereas IFN-α is involved in defense against viral infections. TGF-β is involved in the regulation of cell growth, differentiation and apoptosis. The interactions of these cytokines with the cellular environments in the liver regulate pro-inflammatory and anti-inflammatory activities and maintain homeostasis.
Liver-related diseases
Liver-related diseases include a wide array of diseases. Several environmental and genetic factors are implicated in these diseases; such factors are together termed as exposome [20,21]. Immune-mediated inflammation, toxins and the gut-liver axis pathways are implicated in diseases such as liver fibrosis, cirrhosis, Non-Alcoholic Fatty Liver Disease (NAFLD), Non-Alcoholic Steatohepatitis (NASH) [22-24]. The pro-inflammatory immune mechanisms involved in the pathogenesis of each of these diseases are diverse. In addition to the inflammatory processes, the gut microbiome, which consists of various microorganisms such as bacteria, fungi and viruses, plays an important role in pathogenesis of liver diseases. Gut health is negatively impacted due to improper diet, excess stress, exposure to various environmental toxins and overuse of antibiotics. In the following sections, we discuss the pathogenesis and treatment for fibrosis, cirrhosis, autoimmune liver diseases and viral liver infections.
Liver fibrosis
Liver fibrosis is a pathological process that occurs when there is an accumulation of collagen and other extracellular matrix proteins in the liver tissue [25]. This accumulation leads to the formation of scar tissue, which impairs liver's normal function. The molecular mechanisms that because liver fibrosis involve a complex interplay of various cell types, cytokines, growth factors and extracellular matrix components. One of the main factors that triggers liver fibrosis is the activation of HSCs, which are usually inactive cells in the liver [26]. HSCs are activated by different stimuli such as cytokines like Transforming Growth Factor-Beta (TGF-β) and Platelet-Derived Growth Factor (PDGF), oxidative stress and Damage-Associated Molecular Patterns (DAMPs). The activation of HSCs plays a significant role in this process; in addition, different signaling pathways such as TGF-β/Smad, PDGF receptor and Wnt/ β-catenin pathways participate in HSC activation and collagen synthesis [27]. Similarly, the PDGF receptor pathway induces HSC proliferation and migration, while the Wnt/β-catenin pathway regulates HSC proliferation and differentiation, both of which contribute to HSC activation and collagen synthesis [28]. Activated HSCs secrete excessive amounts of extracellular matrix proteins, including collagen, which leads to the development of liver fibrosis.
Liver fibrosis is also induced by hepatocytes, kupffer cells, NK cells and endothelial cells [29]. These cells produce various cytokines, growth factors and extracellular matrix components that contribute to fibrosis. Kupffer cells produce TNF-α and IL-6, which are proinflammatory cytokines that activate HSCs and trigger collagen synthesis. Hepatocytes, on the other hand, generate cytokines and growth factors like TGF-β, PDGF and Vascular Endothelial Growth Factor (VEGF), which are also involved in liver fibrosis [30].
Liver fibrosis is a result of multiple cellular, metabolic and signaling pathways. Some of the liver fibrosis therapies that are in phase 3 clinical trials are listed in Table 1 [31,32].
| Therapy | Indication | Clinical trial number |
|---|---|---|
| Hydronidone | Liver fibrosis associated with chronic viral hepatitis B | NCT05115942 |
| Candesartan | Liver fibrosis | NCT03770936 |
| Ramipril | ||
| Dexmedetomidine (PRECEDEX™) and ketamine | Liver cirrhosis | NCT05674877 |
| Lanifibranor | Nonalcoholic Steatohepatitis (NASH) | NCT04849728 |
| Faecal microbiota transplantation | Liver cirrhosis | NCT04932577 |
| Growth hormone | Decompensate liver cirrhosis | NCT05253287 |
| Autologous endothelial progenitor cell therapy (CD133) | Reversal of liver cirrhosis | NCT03109236 |
| Simvastatin | Liver cirrhosis | NCT03654053 |
| Allopurinol | Liver cirrhosis, quality of life | NCT05828836 |
Note: Sixteen phase 3 trials were listed on clinicaltrials.gov as of 03 June 2023. The table lists some of the trials for the treatment of liver diseases, ranging from small molecules, protein therapeutics, cell therapy and fecal transplantation.
Table 1: Therapies for liver diseases currently under phase 3 clinical trials.
Liver cirrhosis
The most common causes of cirrhosis are Viral Hepatitis (HV), Non-alcoholic Steatohepatitis (NASH) and Alcoholic Liver Disease (ALD); moreover, Hepatocellular Carcinoma (HCC) occurs in the background of a cirrhotic liver [33]. Liver cirrhosis can be caused by a number of etiological causes, all of which end up triggering processes similar to hepatic fibrosis and impeding organ function [34]. The deterioration of liver cells causes necrosis and as a counteractive measure, an extracellular fibrotic scar material is formed in the regions of necrotized tissue to substitute the parenchyma with regeneration nodules.
HSCs play a central role in the pathogenesis of liver cirrhosis. The activation of HSCs occurs in two stages [33]. The first, termed as initiation or pre-inflammatory stage, is triggered by the products of cellular apoptosis, oxidative stress and signals from kupffer cells, hepatocytes, platelets and endothelial cells. The second, the perpetuation phase, involves cell proliferation and fibrogenesis accompanied by a notable inflammatory response. Metalloproteinases, mainly produced by stellate cells, play a crucial role in this regard. Matrix Metalloproteinases (MMPs) and their inhibitors, tissue inhibitors of metalloproteinase, are proteins that are essential for the breakdown of the matrix. Their presence in liver damage suggests that regular extracellular matrix degradation could be involved in the development of fibrosis [35]. Thus, various MMPs are associated with various stages of liver injury such as disease resolution disease, inflammation, fibrosis, cirrhosis and hepatocellular carcinoma. These enzymes degrade the extracellular matrix and when the production of these enzymes is higher than their rate of degradation, the architecture of the matrix is replaced by fibrotic tissue [35]. Liver fibrosis is eventually an outcome of long-term injury to the organ. The elevated levels of cytokines, extracellular matrix components and breakdown products cumulatively contribute to severe cases of fibrosis, such as bridging fibrosis or cirrhosis. Therapeutic applications have targeted the modulation of dysregulated immune cells and cytokines, which eventually results in the re-absorption of excess degraded matrix and consequently reverses liver damage [26].
Gut microbiome: It is known that liver functions are influenced by gut microbiome. Gut and liver are connected with each other through the hepatic portal vein. The bidirectional relationship between liver and gut is known as gut-liver axis [36]. Besides the hepatic portal vein, gut and liver are connected through several other vessels, including the biliary tract, the portal vein and systemic circulation [37,38]. This close relationship between the gastrointestinal tract and liver has a significant impact on maintaining liver health [39]. Dysbiosis, which is a disruption of the balance of the gut microbiota, increases intestinal permeability. This consequently causes endotoxins to move into the portal vein and activate inflammatory cytokines in the liver. These endotoxins act as pathogen-associated molecular patterns that bind to toll like receptors and trigger an innate immune response with the production of inflammatory cytokines. TLR 4 binds to endotoxins, whereas TLR 2 binds to lipoproteins and peptidoglycan from gram positive bacteria. MMPs and microbiome are linked to fibrosis and are essential for extracellular matrix remodeling [40]. The pathogenesis of cirrhosis and the precise function of gut microbiome are not yet clear. However, some recent studies have shown improvement in liver cirrhosis-induced animals and liver cirrhosis patients by modulating gut microbiome. This highlights the importance of gut microbiome modulation in treating liver diseases, suggesting novel approaches for therapeutic strategies [39]. The modulation of gut microbiome by adopting a healthy diet that helps gut microbial activity and transplantation of fecal microbiome from healthy subjects to promote the growth of good gut microbes may ameliorate dysbiosis in patients and improve their prognosis [41].
Certain bacteria release endotoxins when they die, which triggers an immune response, leading to inflammation. Such frequent exposure to endotoxins may lead to chronic inflammation of the liver. Evidence suggests that misuse of alcohol alters gut composition and intestinal permeability. Liver cirrhosis patients demonstrate gut dysbiosis characterized by the imbalance of Streptococcaceae and Enterobacteriaceae overgrowth, which are potentially pathogenic and decrease in Lachnospiraceae, which are good bacteria [37].
Role of bile: As cirrhosis progresses, plasma endotoxin levels continue to increase and bile acid plays a major role in this process. Primary bile acids are synthesized by liver and are combined with taurine or glycine to be secreted into bile. Thereafter, the synthesized bile is stored in the gall bladder and delivered to the small intestine [42]. Gut microbiome generates secondary bile acids, including deoxycholic acid and lithocholic acid, by deconjugation and dihydroxylation; these bile acids are reabsorbed into the enterohepatic circulation at the ileum [43]. Bile acids are important not only for the absorption of vitamins and dietary fats but also as ligands for the nuclear receptor farnesoid X receptor and the takeda-G-protein coupled receptor [44]. Therefore, close interaction between gut and liver can be a major factor in the pathogenesis of liver damage and liver disease progression.
Liver cirrhosis is a complex disease with multi-factorial etiologies and pathways of disease progression. These pathways involve proinflammatory cytokines, gut microbiome and bile, which lead to liver dysfunction. Several therapies for liver cirrhosis target these pathways [45]. Some of the therapies for liver cirrhosis that are in phase 3 clinical trials are listed in Table 1.
Autoimmune diseases of the liver
OAILDs are chronic inflammatory hepatobiliary disorders that are classically defined to include three distinctive clinical presentations: Autoimmune Hepatitis (AIH), Primary Biliary Cholangitis (PBC) and Primary Sclerosing Cholangitis (PSC) [46,47]. Although autoimmune liver diseases are rare, their clinical burden is disproportionately high relative to their incidence and prevalence in the population. Age, sex and race also impact clinical outcomes, and patient morbidity and mortality are reflected by high demand for gastroenterology, hepatology and organ transplant services. Meaningful changes in disease epidemiology are reported, with increasing incidence and prevalence of AIH and PSC in Europe and rising prevalence of PBC across Europe, North America and the Asia-Pacific region [48]. Without intervention, AILD can lead to complications like decompensated cirrhosis and liver failure as well as Hepatocellular Carcinoma (HCC).
A unique nationwide family study on medically diagnosed patients has highlighted that AIH has familial association with PBC and other autoimmune diseases. It stated that there was a high risk of AIH between spouses, which exceeded the risk between siblings, suggesting the existence of strong environmental risk factors. AIH and PBC were also associated with multiple other AILDs [49]. This makes it imperative to understand the immuno-pathology of AILDs to look for alternate/non-conventional treatment options with better outcomes.
Autoimmune hepatitis: Autoimmune hepatitis is a chronic inflammatory liver disease characterized by elevated levels of aminotransferases, Immunoglobulin G (IgG), specific autoantibodies and interface hepatitis on histology. The annual incidence of AIH has been recorded as 0.67 to 2.0 per 100,000 which varies according to the geographical region and ethnic background. As with most autoimmune diseases, AIH has a female preponderance [50]. Liver Antigen Presenting Cells (APCs) present self-antigens to naïve T cells in the presence of costimulation, which leads to inflammatory responses and immune injury. In addition, transport of antigen-MHC complexes by trogocytosis and extracellular vesicles across cells contribute to the mechanisms of amplified autoimmune responses [51]. Therapies for AIH include generalized immune suppressants, such as corticosteroids, azathioprine, calcineurin inhibitors (sirolimus and tacrolimus) and mycophenolate mofetil. Withdrawal of immuno-suppression often results in disease relapse and in some cases, therapy is ineffective or associated with serious side effects. Imbalance between effector and regulatory cells permits liver damage perpetuation and progression in AIH. Impaired expression and regulation of Cluster of Differentiation (CD) 39, an ectoenzyme key to immunotolerance maintenance, have been reported in tregs and effector T helper (Th) 17-cells derived from AIH patients [52]. Interference with these altered immune-regulatory pathways can open new therapeutic avenues that in addition to limiting aberrant inflammatory responses, would also reconstitute immune homeostasis.
Primary biliary cholangitis: Primary biliary cholangitis is a cholestatic AILD characterized by chronic, destructive non- suppurative granulomatous cholangitis of the small and medium- sized intrahepatic bile ducts. PBC is characterized by the presence of disease-specific Anti-Mitochondrial Antibodies (AMAs) [47,53]. The disease is rare, with an incidence reported to be approximately 0.7 to 49 per million per year. It is predominantly observed in women, with a 10:1 female preponderance. PBC develops in patients with genetic predisposition to autoimmunity in whom epigenetic mechanisms silence the Cl-/HCO3 - exchange in both cholangiocytes and lymphoid cells. Defective Anion Exchanger-2 (AE-2) function can cause Biliary Epithelial Cell (BEC) damage because of decreased biliary HCO3 - secretion. This disrupts the protective alkaline umbrella that normally prevents the penetration of toxic a polar bile salts into cholangiocytes. AE2 dysfunction also causes increased intracellular pH (pHi) in cholangiocytes, leading to the activation of soluble adenylyl cyclase, which sensitizes BECs to bile salt-induced apoptosis. AE2 deficiency can disturb mitophagy in BECs, thus promoting the accumulation of defective mitochondria, oxidative stress and presentation of mitochondrial antigens to the immune cells. As women possess more acidic endo- lysosomal milieu than men, mitophagy might be more affected in women with an AE2-defective background. Apart from this, AE2 downregulation in lymphocytes may also contribute to altered immune-regulation, facilitating autoreactive T-cell responses [54].
The interactive relationship between gut and liver, known as the gut-liver axis, is established by the portal vein, which enables the transport of gut-derived products directly to the liver. PBC can change the gut microbiome by causing intestinal motility disorders, immunologic derangement, bile secretory defects and portal hypertension. The metabolites of these bacterial have significant immunomodulatory activities [55].
Primary sclerosing cholangitis: Primary sclerosing cholangitis is a progressive disease of the biliary tract characterized by inflammation and scarring of intrahepatic and extrahepatic bile ducts, leading to cirrhosis. Approximately 80% of PSC patients suffer from Inflammatory Bowel Disease (IBD), especially ulcerative colitis. PSC occurs more commonly in men and it is typically diagnosed between the ages of 30 and 40 years. Genetic factors contribute about 10% to a predisposition for PSC and may explain the increased risk of PSC in first-degree relatives of PSC patients. More than 20 risk genes predominantly within the HLA complex or associated with IBD and other immune- mediated diseases have been identified in PSC patients in a large genome-wide association study [56]. The mechanisms involved in the pathogenesis involve exposure of hydrophobic bile acid on cholangiocytes. Bile acid is abundant in the gut and it undergoes a bacteria-mediated transformation into bioactive molecules. Its metabolites control the host immune response by modulating the balance of Th17 and regulatory T cells. Primary bile acids and their derivatives are increased in patients with PSC. Alternatively, activated cholangiocytes are correlated with activated biliary tree stem cells that induce biliary fibrosis and progression of the bile duct. TNF-α, TGF-β1, IL-1β and IL-6, along with CD8+ and CD4+ T cells, cause myofibroblast activation and fibrosis. These induce peribiliary fibrosis development and subsequent cirrhosis through interactions with HSCs. Chronic injury can lead to cholangiocyte senescence and differentiation of matrix-depositing HSCs from myofibroblasts and portal fibroblasts, resulting in tissue scarring and bile duct strictures.
PSC being a part of the hepatobiliary manifestation of IBD, gut-derived adaptive and innate immune responses contribute to chronic and progressive biliary inflammation. Studies on the pathophysiology of PSC extended to integrative analysis between gut microbiota, gene expression and immunologic response endorse this observation. Park, et al., [56] reported microbial alteration and differential gene expression (colonic transcriptome) in PSC/IBD and ulcerative colitis patients and implicated the dysregulation of bile acid metabolism.
Therapies for autoimmune hepatitis
The current front-line treatments center on broad immunosuppressive agents and Ursodeoxycholic Acid (UDCA), a biliary protective drug whose mechanism of action is still poorly understood [57]. There is an unmet need for improved treatment options with increased efficacy in hard-to-treat groups, particularly pediatric AIH patients, refractory PBC patients and PSC patients.
Antigen-specific immunotherapy: Antigen-specific immunotherapy has been practiced in the field of allergy for more than 100 years. Increasing interest in the development of antigen- specific approaches for specific immunotherapy of autoimmune conditions follows evidence that treatment of experimental animals with antigens can lead to amelioration of disease. Currently, these approaches target CD4 T-cell recognition of self- antigens. This is because CD4 T-cells control the generation of all of the tissue-damaging mechanisms associated with autoimmunity, including pathogenic autoantibodies, antigen-driven inflammation and self-antigen specific CD8 T-cells [57]. A few of the antigen- specific immunotherapies under trials for the treatment of liver autoimmune diseases have been reviewed elsewhere [58].
B cell and T cell therapies: Several novel therapies are under investigation for immune-mediated liver diseases that directly or indirectly target the B cell lineage [59]. These have various mechanisms of action including B cell depletion, inhibition of direct B cell signaling through cell-cell interactions and inhibition of B cell signaling through cytokine production. Agents that directly target B cells include monoclonal antibodies specific for the CD19 receptor. The treatment leads to depletion of CD19- expressing cells, including B cells and pre-B cells. Monoclonal antibodies alter B cells or pre-B cell surface receptor co-engagement with receptors on other cells. These include interruption of T cell activation by antigen presenting B cells through blocking CD80/86- CD28 signaling and suppression of B cell responses through co- engagement of the Fc-gamma receptor IIb with CD19 [60].
Viral hepatitis
According to the World Health Organization (WHO), about 325 million people suffer from chronic hepatitis infection. There are five main strains of the hepatitis virus: A, B, C, D and E. Each strain differs in the mode of transmission, severity of illness, geographical distribution and prevention methods [61]. Hepatitis A Virus (HAV) and Hepatitis E Virus (HEV) are responsible for a large proportion of cases of acute hepatitis, while Hepatitis B Virus (HBV) and Hepatitis C Virus (HCV) are the most common causes of chronic viral hepatitis. Hepatitis D Virus (HDV) is a satellite virus that requires the envelope proteins of HBV for cell release and uptake [62,63].
The host innate immune system forms the first line of defense against hepatitis viruses. Hepatitis viruses are sensed by specific Pathogen Recognition Receptors (PRRs) that subsequently trigger the innate immune response and Interferon (IFN) production. However, hepatitis viruses evade host immune surveillance via multiple strategies, which help compromise the innate immune response and create a favorable environment for viral replication [64].
Hepatitis A
HAV is a positive-stranded Ribonucleic Acid (RNA) virus within the hepatoviral species. It is an ancient hepatotropic pathogen belonging to the Picornaviridae family that has been infecting humans for millennia. HAV mainly, but not exclusively, transmits through the fecal-oral route and causes epidemics. In addition, it is responsible for sporadic, anicteric or icteric hepatitis. The epidemiology of HAV is complex and is shifting in countries that are making improvements in public health and sanitation. Understanding the mechanisms involved in the pathogenesis of HAV infection-induced liver dysfunction is critical to developing novel therapies. Endosomal gangliosides have been recently implicated as essential receptors for HAV. After intracellular genome translation, polyprotein processing and replication, the virus generates an immune-mediated inflammatory process against infected hepatocytes. HAV is then released into the blood encased in a protective host-derived membrane termed eHAV, which shields the circulating virus from host’s neutralizing antibodies [65].
Vaccination against HAV is effective in preventing the disease [66]. Immune memory against HAV following injection of the vaccine persists for years. As shown for the formaldehyde-inactivated vaccines, immune memory lasts at least for 22 years without the necessity of a booster dose [67]. However, countries where HAV is endemic need to closely monitor its epidemiology and disease burden of Hepatitis A to allow timely identification of transition toward intermediate endemicity and introduction of preventive strategies.
Hepatitis B
HBV is a small Deoxyribonucleic Acid (DNA) virus; it is non- cytopathic and hepatotropic and belongs to the Hepadnaviridae family. It has the potential to cause a persistent infection, ultimately leading to cirrhosis and hepatocellular carcinoma.
Innate and adaptive immune cells play crucial roles in controlling HBV infection. These cells are also accountable for inflammation, which eventually results in liver pathologies. During the initial phase of HBV infection, innate immunity is triggered, leading to antiviral cytokine production. Subsequently, adaptive immune system is activated and its components are recruited inside the liver, resulting in successful virus elimination. In chronic HBV infection, significant alterations occur in both innate and adaptive immunity. These include expansion of regulatory cells, overexpression of co- inhibitory receptors, presence of abundant inflammatory mediators and modifications in immune cell-derived exosome release and function. These alterations overpower antiviral response, leading to persistent viral infection. Subsequent immune pathologies associated with disease progression follow and may present as fibrosis, cirrhosis and/or hepatocellular carcinoma [68].
The current approach in the treatment of chronic HBV infection is to use a combination of multiple drugs including a backbone of a nucleos(t)ide analogue, one or more new direct-acting antiviral drugs and at least one immuno-modulator [69]. The aim of therapeutic vaccines is to stimulate the host immune response to restore HBV-specific immune control while suppressing HBV replication and ultimately inducing Hepatitis B Surface Antigen (HBsAg) loss [70]. Alternatively, immunotherapy with vaccines and checkpoint inhibitors can boost T cell functions in vitro and therefore may be used to reinvigorate the impaired HBV-specific T cell response.
Hepatitis C
HCV is a member of the Hepacivirus genus in the family Flaviviridae. The only known hosts are humans and chimpanzees [71]. HCV RNA acquires mutations that allow the virus to escape the host immune response, which contributes to the diversity and evolution of the HCV genome. Immune cell detection of HCV activates signaling pathways that produce interferons and trigger the innate immune response against the virus, preventing HCV replication and spread. Despite immune activation, HCV can evade the host response and establish persistent infection.
The current HCV vaccine development is mostly focused on weakly immunogenic subunits, such as surface glycoproteins or non-structural proteins. Complex adjuvants that stimulate different components of innate immunity are being developed to enhance their antigenicity. Several studies are in progress to determine the immunological determinants against viral clearance, persistence and protective immunity [72]. The aim of an effective vaccine is to target humoral immune responses to induce broadly neutralizing antibodies in protection against HCV infection [73].
Hepatitis D
Hepatitis Delta Virus (HDV) is a human pathogen and it is the only known species in the genus Deltavirus [74,75]. It is a satellite RNA virus that depends on HBV for propagation. It utilizes HBsAg as a viral envelope and shares the same hepatocyte receptor for viral entry. HDV is among the smallest of viruses capable of causing human disease, yet HBV co-infection with HDV is the most severe form of viral hepatitis. About 5% of individuals infected with HBV have experienced HDV infection, with higher prevalence in certain geographic areas and populations.
Innate immune responses that induce IFNs are the main mediators of early containment of viral replication. Untreated HBV/HDV co-infected patients tend to have even higher peripheral NK cell frequencies than patients with other hepatitis virus infections. Anti- HDV antibodies are detectable in low titers in acute-resolving HDV infection, but at higher titers in persistent infection. In patients with active hepatitis, anti-HDV Immunoglobulin M (IgM) often persists at high titers [76].
The ideal endpoint for any anti-HDV therapy would be HBsAg loss with anti-HBs seroconversion. Elimination of replicating HDV RNA from the liver is demonstrated by conducting Polymerase Chain Reaction (PCR) on serum or plasms samples at least 24 weeks after treatment discontinuation. New therapeutic approaches include the use of RNA interference and antisense oligonucleotides, which lead to substantial decline in HBsAg levels after a few weeks of administration in the absence of pegylated- Interferon (peg-IFN) [77].
Hepatitis E
HEV is a small, non-enveloped, positive-sense single-stranded RNA virus. It is classified as a species of Orthohepevirus A in the Orthohepevirus genus. It is a leading cause of acute viral hepatitis worldwide [78]. The infection is transmitted by the fecal-oral route via contaminated water and it is usually asymptomatic or self‐limiting in the general population. However, acute infection in pregnant women may cause severe clinical outcomes, including fulminant hepatic failure with high mortality rate reaching up to 20%-30%. Recent revisions have proposed eight genotypes and 36 subtypes that infect mammals, with genotypes 1-4 being a major cause of disease in humans [79].
HEV-induced host immune response is an important mechanism leading to hepatic cytopathic injury. Analysis of the Peripheral Blood Mononuclear Cells (PBMCs) of patients with AHE showed that the number of NK and NKT cells in these patients was significantly lower than that in the healthy control group, but the number of activated NK and NKT cells in these patients was significantly higher than in the healthy control group [80]. The increase in the number of cells illustrates the possible acute HEV infection because the activated NK and NKT cells might migrate from peripheral blood to infiltrate the liver and play a role in killing viruses in the liver. The upregulation of IFN-γ and TNF-α in the liver of Hepatitis E Virus-Acute Chronic Liver Failure (HEV-ACLF) patients is not offset by IL-10 and the imbalance of the expressions of pro-inflammatory cytokines and anti-inflammatory cytokines in the liver of patients is the important immune mechanism of HEV-ACLF liver injury.
Ribavirin therapy for a period of three months is the drug of choice for severe acute hepatitis, acute-on chronic liver failure and chronic infections from hepatitis E virus in immuno-compromised patients who are unresponsive to decreased immunosuppression. Pegylated interferon α is used for ribavirin-resistant liver transplant patients with chronic hepatitis E. A vaccine for HEV is now available in China [78].
Role of immuno-modulators in functional cure strategies for hepatitis
A major challenge in the development of therapies against viral hepatitis is overcoming skewed virus-specific immune response due to decades of chronic hepatitis infections [81]. Nucleos(t)ide analogues are highly effective at reducing viral load and resolving liver damage, which is important in the progression of disease, but they do not target cccDNA. These agents do not achieve cure. Activation of anti-virus immune response is required for effective viral control, which has been demonstrated in natural course of hepatitis infection.
There are four primary categories of treatment strategies currently in development [82].
• Therapeutic vaccines that target virus-specific T and B-cell responses.
• Innate immune-modulators that target pattern recognition receptors such as Toll-Like Receptors (TLRs), Stimulator of Interferon Gene (STING) and Retinoic Acid-Inducible Gene I (RIG-I).
• Checkpoint inhibitors.
• Immunotherapies consist of anti-HBs monoclonal antibodies. These have the potential to bind circulating HBs antigen in the serum and potentially target it to immune cells, such as dendritic cells, monocytes and macrophages, which could use it to boost T- and B-cell responses [83].
Liver-related diseases cause significant morbidity and mortality. We have detailed the recent advances in understanding normal liver functions and the immune-pathogenesis of liver diseases in fibrosis, cirrhosis, autoimmune diseases and infectious diseases. The multi-factorial mechanisms involve various aspects of innate and adaptive immune responses mediated by inflammatory and regulatory cytokines and receptor–ligand interactions. These induce activation of specific signal transduction pathways and culminate in transcription of genes. Understanding these immune-regulatory mechanisms in the pathogenesis of liver fibrosis, cirrhosis and autoimmune diseases is critical in developing novel therapies. To understand the hallmarks of health and maintaining a healthy lifestyle, we have reviewed the recent advances in the immunology of liver, with an emphasis on the functioning of normal livers and pathogenesis of fibrosis, cirrhosis, autoimmune diseases and viral hepatitis. Accumulation of research has indicated the association of the gut-liver axis with the pathogenesis of liver diseases. A few phase 3 clinical trials are addressing the dire need for novel treatments. There is an opportunity for utilizing immunotherapies, cell and gene therapies and synthetic biology-based therapies to target gutliver- based pathologies for potentially reversing liver pathogenesis, given the regenerative potential of this organ. There is a need for longitudinal studies by collecting Genome-Wide Association Study (GWAS), multi-omics and high dimensional measurements to guide personalized therapies.
We thank Vihang Ghalsasi, PhD for detailed copy editing and providing useful comments for the structure and flow of the manuscript. Pranoti Mandrekar for advice on the context, and Abhishek Kulshrestha for helping with making the connections.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
Citation: Bakhale C, Chirmule N, Singh P, Gandhi P, Mukherjee S, Kar S (2024) Immunology of the Liver in the Pathogenesis of Fibrosis, Cirrhosis, Autoimmune Disease and Viral Hepatitis. Immunotherapy (Los Angel). 10:243.
Received: 23-Feb-2024, Manuscript No. IMT-24-29758; Editor assigned: 26-Feb-2024, Pre QC No. IMT-24-29758 (PQ); Reviewed: 11-Mar-2024, QC No. IMT-24-29758; Revised: 18-Mar-2024, Manuscript No. IMT-24-29758 (R); Published: 25-Mar-2024 , DOI: 10.35248/2471-9552.24.10.243
Copyright: © 2024 Bakhale C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.