Journal of Medical Diagnostic Methods
Open Access
ISSN: 2168-9784
ISSN: 2168-9784
Research Article - (2017) Volume 6, Issue 1
Tenofovir disoproxil fumarate (TDF) remains the most widely use and prescribed first line antiretroviral therapy in the treatment of patients infected HIV-infection but unfortunately it is possibly nephrotoxic especially on the kidney tubules. In this longitudinal study we evaluated the influence of TDF on the renal tubules, measuring urinary phosphate and protein. 57 patients infected with HIV were recruited and grouped as follows: TDF group (21 patients), Non-TDF group (21 patients) and treatment naïve group (15 patients). Indicators of renal tubular injuriousness together with other common biomarkers of renal injury were evaluated. Phosphaturia was defined as urinary phosphate 20.0 mg/dl and the prevalence of phosphaturia after 12 weeks of follow-up were as follows: TDF group (8), Non-TDF group (1) and treatment naïve group (3). Proteinuria was defined as positive protein on dip stick urine and the prevalence among the different ART regimen groups after 12 weeks were as follows: TDF group (4), Non-TDF group (1) and treatment naïve group (1). CD4 count for the different regimen groups after 12 weeks were as follows: TDF (659.95 ul/cells/<50 copies/ml), non-TDF (363.24 ul/cells/<500 copies/ml) and treatment naïve (276.63 ul/cells/<1000 copies/ml). This higher incidence in phosphaturia and proteinuria in the presence of increased CD4 counts in HIV patients exposed to TDF is believed to be progressive and may subsequently result in a generalized renal tubular toxicity before any rise in serum creatinine.
Keywords: Tenofovir disoproxil fumarate; Tubular toxicity; Phosphaturia; Immunopotentiation
Chronic kidney disease (CKD) is common in HIV-infected individuals, and is associated with proteinuria and phosphaturia (which are increase in phosphate concentration) [1]. Urinary markers of tubular injury such as urinary calcium, phosphate, uric acid, etc. have been associated with higher kidney disease risk in HIV patients, however early diagnosis and management of patients with kidney toxicity in the main care scenery are most essential to maintaining good health, improved quality of life and again improve treatment outcome in HIV-infected patients with renal disorders.
Phosphate is an essential buffer, mainly in the urine and it basically contributes to the maintenance of acid-base balance. It is used in the investigation/monitoring of hyper-phosphataemia, (e.g. in chronic kidney disease (CKD) or in conditions resulting in cell death). Urine phosphate is mostly measured and evaluated together with serum phosphate, as well as urine and serum creatinine, in order to calculate the renal tubular reabsorption of phosphate (TmP/GFR), which is used to investigate the possible cause of hyperphosphatemia and hypophosphatemia. HIV patients are prone to renal injury which is known to be the major cause of phosphaturia [2]. Again a speedy and sharp increase in plasma phosphate can sometimes result in hypocalcaemia (decrease in calcium level) in HIV patients resulting in skeletal abnormalities [3,4]. HIV-infected patients seem to have additional causes of low serum phosphate concentration compared with the general population [5], such as; Hypovitaminosis D: in literature there is a great evidence of this condition related to HIV status. Several factors have accounted for this such as the invitro demonstrated effect of reduction of the 25-and 1-hydroxylation by protease inhibitors [6], in literature a roles of efavirenz and tenoforvir has been proposed as well [7]. Since HIV and some antiretroviral drugs are nephrotoxic, and more specifically tubulotoxic, an evaluation of parameters more specific to the renal tubules would be of great clinical usefulness.
Study population
HIV-positive ART-unexposed patients were enrolled in this study starting from October 2015 to June 2016 at the antiretroviral/HIV clinic of the University of Port Harcourt Teaching hospital, a Tertiary Hospital for the treatment of infectious diseases located in the capital State, Port Harcourt, Nigeria. Patients with severe or terminal clinical conditions or complete organ failures were exempted from the study. Furthermore, patients with reasonable to severe renal disorders or nephrotic syndrome were not recruited/enrolled in the study. The patients were furthermore divided in to three major groups according to drug experience. The Group 1, comprised patients who received tenofovir-based regimen (TDF group) while Group 2 received nontenofovir (non-TDF)-based regimen (i.e. niverapine, zidovudine, lamivudine, emtricitabine, efavirenz.. etc) and Group3 were HIVtreatment naïve patients whose CD4 counts were higher than 500 ul/ml. Clinical features of each individual patient were collected from the clinical record. Immuno-virological status and CD4 cell count at baseline was also recorded. Fifty seven patients completed the study and renal tubular pathophysiological parameters were made readily accessible from these patients. All patients gave informed consent to be a participant in the study.
Study protocol and specimen collection
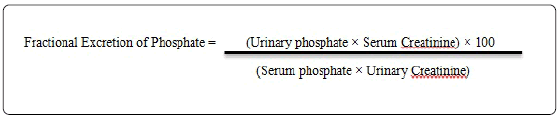
The study period was 12 weeks. Both urine and Serum samples were gotten at different times but most notably at baseline (Visit 1), after 4 weeks (Visit 2), and 12 (Visit 3) weeks of treatments with antiretroviral therapy with any one of the two NRTI- based regimes. A starve yourself blood sample was obtained during the patients visits; the patients were made also to provide 24 hours urine sample with a morning spot urine sample. Blood samples were accessed for predictable chemistry parameters and a complete blood count. The 24 hours urine samples collected were then centrifuged and referred to the central laboratory for assessment of routine parameters such as protein, and phosphate etc. In order to eradicate the effect of inconsistent urine collection, all parameters to be assayed were expressed as ratio versus urinary creatinine. The spot urine samples collected were centrifuged and stored at -70°C until being ready to be tested for the desirable urinary markers. Fractional Excretion of Phosphate was calculated using the formular below:
Carley et al., [8] simple normograms was used for the calculation of sample size for this clinical diagnostic study.
Statistical analysis
Continuous and categorical data are given as mean and standard deviation and percentages respectively. All the groups of patients analyzed were first matched using chi-squared for categorical data and parametric tests for continuous variables. The level of significance was 0.05. All statistical analyses were accomplished using SPSS Version 20.0 software package (SPSS inc, Chicago, Illinois, USA).
This study was carried out in agreement with the Helsinki Declaration and begins only after authorization was given by the University of Port Harcourt Teaching Hospital Ethical Committee (UPH/R&D/REC/04) and the University of Port Harcourt Ethics Committee (UPTH/ADM/90/S.II/VOL.X/454). All data produced were kept in firm privacy.
Characteristic of patients study
A total of Ninety eight patients were enrolled in to the study from June 2015 to October 2016. Complete data were gotten from a total of 57 patients while 41 patients were lost to follow-up due to nonadherence to study protocol. Of the 57 patients, 42 patients started a first line antiretroviral therapy i.e. 21 Patients were on TDF while 21 patients were on Non-TDF Antiretroviral therapy. During the study period all patients met inclusion criteria. Among these 57 enrolled patients, 21 (36.8%) initiated tenofovir-containing regimen, 21 (36.8%) initiated tenofovir-sparing regimen and 15 (26.4%) were antiretroviral Naïve patients. Baseline socio-demographic paramters and laboratory features were compared among the groups.
The mean ages of male and female participants were: 36.43 and 30.97 years respectively, with a statistically significant difference of P = 0.001.
Mean Serum Creatinine (mmol/L) (standard deviation): [TDF 107(2.81), Non-TDF 93.11(2.10) and Drug-Naïve 112.25 (3.84)]. The difference between the regimen groups was statistically significant (P=0.001, ANOVA).
Mean Serum Phosphate (mmol/L) (standard deviation): [TDF 1.03 (0.03), Non-TDF 1.12(0.03) and Drug-Naïve 1.56 (0.003)]. The difference between the regimen groups was statistically significant (P=0.000, ANOVA).
Phosphaturia was defined as values of fractional excretion of phosphate ≥ 20.0 mg/dl. At commencement there was no significant difference (X2=1.745, P=0.418), between the regimen groups. Phosphaturia was absent in the TDF and Naive groups with only 1(1.8%) patient presenting with phosphaturia in the N-TDF group (Table 1).
| Phosphaturia (Naïve group) | Phosphaturia (N-TDF group) | Phosphaturia (TDF group) | Total % | ||||
|---|---|---|---|---|---|---|---|
| Absent | Present | Absent | Present | Absent | Present | ||
| Before Commencement | 15 (26.3%) | 0 (0.0%) | 20 (35.1%) | 1 (1.8%) | 21 36.8%) | 0 (0.0%) | 1 (1.8%) |
| After 4 weeks | 14 (24.6%) | 1 (1.8%) | 17 (29.8%) | 4 (7.0%) | 18 31.6%) | 3 (5.3%) | 8 (14.0%) |
| After 12 weeks | 12 (21.1%) | 3 (5.3%) | 20 (35.1%) | 1 (1.8%) | 13 22.8%) | 8 14.0%) | 12 (21.1%) |
Table 1: Prevalence of phosphaturia in the different ART-groups at different time points. Phosphaturia was defined as fractional excretion of phosphate ≥ 20.0 mg/dl.
Table 1 also shows the prevalence of phosphaturia after 4 weeksn. After 4 weeks (visit 2) of exposure to antiretroviral therapy, there was a remarkable increase in phosphaturia among the groups; N-TDF group 4 (7.0%), antiretroviral Naïve group 1 (1.8%) and TDF group 3 (5.3%). The difference between the groups was not statistically significant (X2 =1.113, P=0.573) and after 12 weeks (visit 3) of exposure, the number of patients with phosphaturia was higher in the TDF group 8 (14.0%), followed by the Naïve group 3 (5.3%) and N-TDF group 1 (1.8%). The difference between the groups was statistically significant (X2=7.033, P=0.030). A total of 12 (21.1%) patients presented with phosphaturia after 12 weeks.
Proteinuria was defined as positive protein on dipstick urine. Table 2 shows proteinuria at commencement, after 4 weeks and after 12 weeks of ART. At commencement the difference between the groups was not statistically significant (X2=0.596, P=0.7742). Prevalence of proteinuria in the different study groups were as follows; TDF group 3 (5.3%), N-TDF group 3 (5.3%) and 1 (1.8%). Prevalence of proteinuria at commencement was 7 (12.3%). After 4 weeks (visit 2) of follow-up, there was a reduced incidence of proteinuria in all three groups with a total prevalence of 5 (8.8%). The prevalence of proteinuria in the different regimen groups was observed to be; TDF group 2 (3.5%), NTDF group 2 (3.5%) and Naïve group 1 (1.8%). The difference between the different regimen groups was not statistically significant (X2=0.113, P=0.945). After 12 weeks (visit 3) of exposure to ART, the number of patients with proteinuria was higher in the TDF group 4 (7.0%), compared with Naïve and N-TDF groups: [1 (1.8%) and 1(1.8%) respectively]. The difference between the groups was not statistically significant (X2=2.597, P=0.273). The total number of patients presenting with proteinuria after 12 weeks was 6 (10.5%). Table 3 shows the CD4 counts/viral load of the different regimen groups to be as follows: TDF group [before ART, (200.45/<1000 copies/ ml), after 4 weeks (261.45/<1000 copies/ml), after 12 weeks (579.12/<50 copies/ml)], Non-TDF group [before ART, (208.28/<1000 copies/ml), after 4 weeks (269.28/<1000 copies/ml), after 12 weeks (343.24/<500 copies/ml)] and Naïve group [before ART, (634.61/<50 copies/ml), after 4 weeks (405.30/<500 copies/ml), after 12 weeks (293.42/<1000 copies/ml)].
| Proteinuria (Naïve group) | Proteinuria (N-TDF group) | Proteinuria (TDF group) | Total % | ||||
|---|---|---|---|---|---|---|---|
| Absent | Present | Absent | Present | Absent | Present | ||
| Before Commencement | 14 (24.6%) | 1 (1.8%) | 18 (31.6%) | 3 (5.3%) | 18 (31.6%) | 3 (5.3%) | 7 (12.3%) |
| After 4 weeks | 14 (24.6%) | 1 (1.8%) | 19 (33.3%) | 2 (3.5%) | 19 (33.3%) | 2 (3.5%) | 5 (8.8%) |
| After 12 weeks | 14 (24.6%) | 1 (1.8%) | 20 (35.1%) | 1 (1.8%) | 17 (29.8%) | 4 (7.0%) | 6 (10.5%) |
Table 2: Prevalence of proteinuria in the diferent ART-groups at different time points. Proteinuria was defined as positive protein on dipstick urine.
| CD4 (ul/cells) | Drug Naïve | N-TDF | TDF | Differences among groups | (Z-test) p-Value |
|---|---|---|---|---|---|
| Means/VL | Means/VL | Means/VL | (ANOVA P-Value) |
||
| Before commencement | 634.61/<50 copies/m | 208.23/<1000 copies/m | 200.48/<1000 copies/m | 0 | 1 versus 2 p=0.000, |
| 1 versus 3 p=0.000, | |||||
| 2 versus 3 p=0.618. | |||||
| After 4 weeks | 405.30/<500 copies/m | 269.78/<1000 copies/m | 261.45/<1000 copies/m | 0 | 1 versus 2 p=0.000, |
| 1 versus 3 p=0.000, | |||||
| 2 versus 3 p=0.585. | |||||
| After 12 weeks | 293.42/<1000 copies/m | 343.24/<500 copies/m | 579.12/<50 copies/m | 0.001 | 1 versus 2 p=0.002, |
| 1 versus 3 p=0.005, | |||||
| 2 versus 3 p=0.023. |
Table 3: Mean CD4 of the study population within 12 weeks. VL=Viral load.
Several cross sectional and longitudinal studies have indicated that Tenofovir disoproxil fumarate (TDF) is not precisely toxic to the glomerulus. Nonetheless, several case study and case evaluation have described more severe cases of kidney involving tubular dysfunction associated with TDF exposure [9,10]. Jerome et al., [11] have revealed that Tenofovir-associated tubular toxicity is the most common sole diagnosed reason for HIV-associated referral to tertiary Hospital facility, accounting for over 20% of Hospital visitation in a single year. The focal site of this toxicity appears to be on the proximal tubule of the kidney, and in a more severe conditions, patients can develop a renal condition refers as Fanconi syndrome which is mostly characterized by renal tubular proteinuria, glycosuria, uricosuria, phosphaturia, and bicarbonate wasting [9,12,13], or severe kidney toxicity. It is worth remarking that estimated glomerular filtration rate (eGFR) solely rely on plasma or serum creatinine measurements, which, is being filtered by the renal glomerulus and then it is secreted across the proximal tubule. Hence, proximal tubular dysfunction (PTD) can result to apparent to moderate changes in the estimated glomerular filtration rate (eGFR). Essentially the capability to detect an early renal dysfunction is principally important in the HIV-infected population basically because HIV-associated muscular damage makes creatinine-based eGFR less precise and again HIV-associated therapies can be evidently nephrotoxic especially in blacks which have been shown to have an 11-fold increased risk of developing chronic kidney disease (CKD), as compared to their white counterparts [14]. Studies indicating severe TDF-associated renal tubular dysfunction are quite rare in our environment but a comprehensive prospective study of HIV-infected patients beginning a TDF treatment described Fanconi syndrome to have occurred in less than 0.1% [15], but it is now very evident that such subclinical tubular abnormalities are now much more common in our population. Nonetheless, further experimental studies have confirmed the role of TDF in prompting significant mitochondrial dysfunction besieged at the renal proximal tubular cells, and laving other very important metabolically energetic cells like hepatocytes [16,17]. A high incidence of related tubular proteinuria has been reported beforehand in patients infected with HIV even before the use of tenofovir (TDF) [18-20], perhaps reflecting the injuriousness of both the HIV-virus and other practices of Antiretroviral therapy used. Additional finding in this study shows a significant difference in mean CD4 levels among the collections. It was therefore detected that there was a rise in the mean CD4 level (<50 copies/ml) in the TDF group related to the Non-TDF and naïve group (<1000 copies/ml and <500 copies/ml respectively). This reflection recommends that notwithstanding been at greater risk of emerging renal toxicity, they have an enhanced immunological outcome. Two major limitations of this study includes: loss to follow due to nonadherence to study protocol and again dipsticks proteinuria was use in measuring albuminuria.
Our data show that treatment of HIV patients with a TDF-based regimen caused a tremendous increase in the CD4 count compared to non-TDF regimen and that continued exposure of the patients to TDFbased therapy despite increase in CD4 count may produce severe adjustments in renal function parameters (such as proteinuria and phosphaturia) after a 12-week treatment period.
I acknowledged the study participants, and Njoku Bestman, for technical assistance.