Journal of Probiotics & Health
Open Access
ISSN: 2329-8901
ISSN: 2329-8901
Research Article - (2024)Volume 12, Issue 2
Calcium deficiency can occur throughout life. In children, calcium deficiency can manifest in its most serious forms, such as rickets and fractures and as osteoporosis in adulthood. The findings from animal models suggest that prebiotic supplementation has an impact on bone mineral density. The objective of this study was to evaluate the effect of prebiotics on bone mineral metabolism in murine models as reported in the scientific literature. A systematic review of the literature was carried out following international quality lines. The full texts were analyzed using the guidelines of the ARRIVE guide; the Risk-of-Bias (RoB) tool for intervention studies according to the SYRCLE. Twelve studies that met the inclusion criteria were included in the meta-analyses. The BMD of the spine showed a positive response to supplementation with prebiotics Standardized Mean Difference (SMD= 0.38, 95% Confidence Interval (CI), -0.29 to 1.04, p ≤ 0.0001). The BMD in the tibia showed the same trend (SMD= 0.87, 95% CI -0.08 to 1.82, p ≤ 0.0001). The calcium content in the femur (SMD= 15.78 95% CI, 5.69 to 25.87, p ≤ 0.0001) was greater in supplemented animals than in the no supplemented animals, as was the magnesium content (SMD= 136, 95% CI, 0.34 to 2.38, p ≤ 0.0001). In conclusion, supplementation with prebiotics has positive effects on bone mineral metabolism, specific to the amount or type of prebiotic, improves bone density and controls reabsorption.
Prebiotics; Mineral metabolism; Bone Mineral Density (BMD); Murine model
ARRIVE: Animals in Research: Reporting In-Vivo Experiments; RoB: Risk of Bias; PRISMA: Preferred Reporting Items for Systematic reviews and Meta-Analyses; GOS: Galacto-Oligo Saccharides; FOS: Fructo- Oligo Saccharides; CFU: Colony-Forming Units; Ca: Calcium; Mg: Magnesium; P: Phosphorus; BMD: Bone Mineral Density; DXA: Dual energy X-ray Absorptiometry
Prebiotics are ingredients that may be naturally contained in food but that can also be added. Prebiotics produce changes in the activity of the intestinal microbiota, providing benefits for the health of the host [1].
Prebiotics are very diverse, including inulin, oligo fructose, Fructo Oligo Saccharides (FOS), Galacto Oligo Saccharides (GOS), soy oligosaccharides and resistant starches, sugar alcohols [2-5].
The health effects of prebiotics have been reported in different studies on animal and human models of bone mineral health. Studies in animal models have shown the positive effects of prebiotics on the absorption and metabolism of minerals (especially calcium and magnesium) and bone composition and architecture. Specifically, murine models are useful because their results can be extrapolated to bone health in humans. Some of these studies have been carried out in growth and postmenopausal models [6,7].
Prebiotic supplementation, usually GOS and FOS, alone or in combination, significantly improves bone mineral density [8,9].
The objective of this systematic review was to evaluate the effect of prebiotics on bone mineral metabolism in murine models.
Research question
What is the effect of prebiotic supplementation on bone mineral metabolism in murine models?
Literature search
A systematic search of the literature in virtual health libraries (Medline, Scielo, Lilacs, DOJA) was carried out following the international quality lines, without language limits or limits on the years of publication. The following search parameters were used: "prebiotics", "bone density" and "mineral metabolism". The search was limited to animal models and the Boolean operator and was used to combine the search terms as follows: ("prebiotics" (MeSH Terms) and "bone density" (Mesh) and mineral metabolism and "animals" (MeSH Terms: noexp).
Study selection process
The title and summary of each of the studies that were identified by the search were evaluated by two reviewers (DGL and ARH); duplicate articles were discarded, as were those that did not meet the inclusion criteria.
Eligibility criteria
Experimental studies in murine models (i.e., rats and mice) that analyzed the effect of supplementation with one or more prebiotics and reported the following as primary results were included: biochemical markers of calcium metabolism and/or bone mineral density. Studies were excluded if they evaluated the combined effects of prebiotics and probiotics or iso-flavonoids or some other component within the supplement that could confound the results and if they reported the results in units that could not be used to compare the results with those of the other studies. The main variables considered were calcium metabolism at the biochemical level and at the level of bone mineral density.
Data extraction
An evaluation of the quality of the studies was performed as follows: Once the title and summary review were completed, the full texts were analysed using the guidelines of the Animals in Research: Reporting In-vivo Experiments (ARRIVE) guide [10]. This analysis was performed by two reviewers (DGL and ARH); when there was any disagreement regarding the inclusion of an article, it was resolved by a third reviewer (JTF). The risk of bias analysis was performed with the SYRCLE RoB tool for intervention studies in laboratory animals [11].
The statistical and forest analyses were carried out with the Stata 12 statistical package for Mac. When there were at least two studies available that reported the results of interest, the meta-analysis was carried out. Studies that did not report complete statistical information were excluded in this analysis. The technique used was for standardized mean differences with a random effects model, given the anticipated heterogeneity of the included studies. When the studies had more than one intervention group, they were compared with the control group. Heterogeneity was evaluated between studies with I² [12].
A total of 45 studies were obtained in the systematic search after the review of titles and abstracts; duplicates, systematic reviews and studies in models of other species (a total of 12 studies) were excluded. Shows the preferred reporting items for systematic reviews and meta-analyses flow chart [13] (Figure 1).
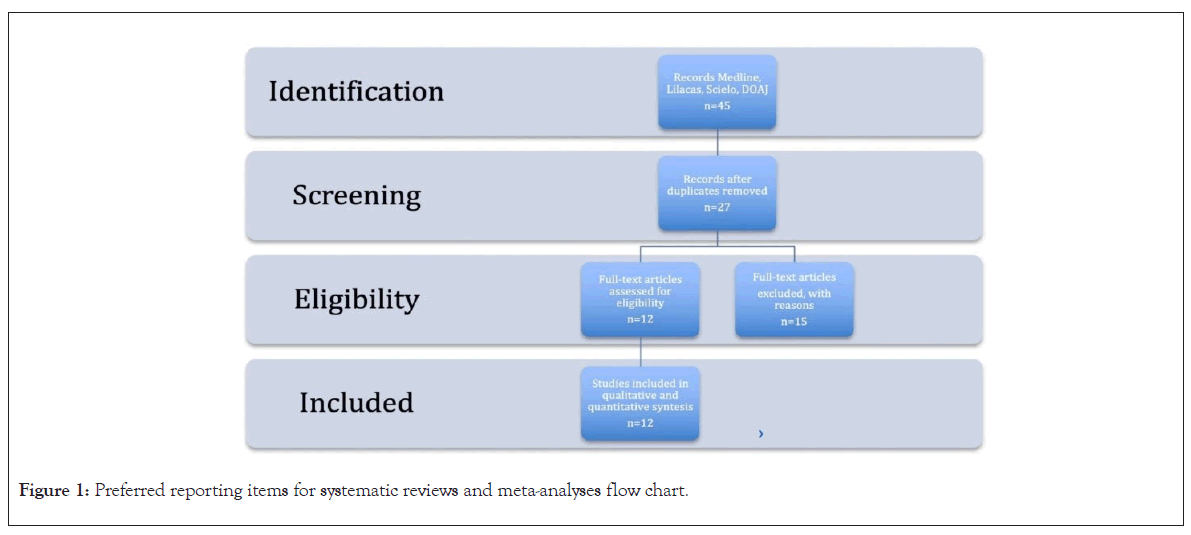
Figure 1: Preferred reporting items for systematic reviews and meta-analyses flow chart.
The prebiotic supplementation included in the studies was within the range of 2 g/kg to 8 g/kg of food and the period of supplementation was from 28 days to 82 days. The details of each study are described, the analysis and data extraction were divided by the animal model that was used: growth or menopause.
The main comparison of most of the studies was with a placebo group, but some studies included, in addition to the placebo group, a comparison with different doses of the same prebiotic or with the combination of two types of prebiotics; so, the meta-analyses were assembled depending on the variables reported by the studies.
Quality evaluation of selected publications. The number of ARRIVE criteria reported by the selected studies. The average score for all included studies was 27.4 ± (1.70), 36 being the maximum possible score. Regarding the individual criteria, there are deficiencies in the report in most of the articles where it evaluates: Sample size, allocation of animals, baseline data of animals and adverse events details.
The results of the bias risk assessment of the included studies: The 12 included studies adequately cover the actual points to the results. Only 9 of the 12 included studies report the baseline characteristics of the study population. Only 3 of the twelve included studies report the random sequence. The other points that the tool evaluates are not reported or the quality of the report is low.
We consider Bone Mineral Density (BMD) of the spine to be the main result in this review. Supplementation with prebiotics and prebiotics plus calcium had a positive effect on BMD, which shows that the greater the amount of prebiotics, the better the BMD (SMD=0.38, 95% CI, -0.29 to 1.04, p ≤ 0.0001) and this effect remained in the same if calcium was included in the supplementation (Figure 2A). The BMD of the tibia was also considered a main result and showed a dependent relationship: The greater the amount of prebiotic, the higher the BMD (SMD=0.87, 95% CI, -0.08 to 1.82, p ≤ 0.0001) (Figure 2B).
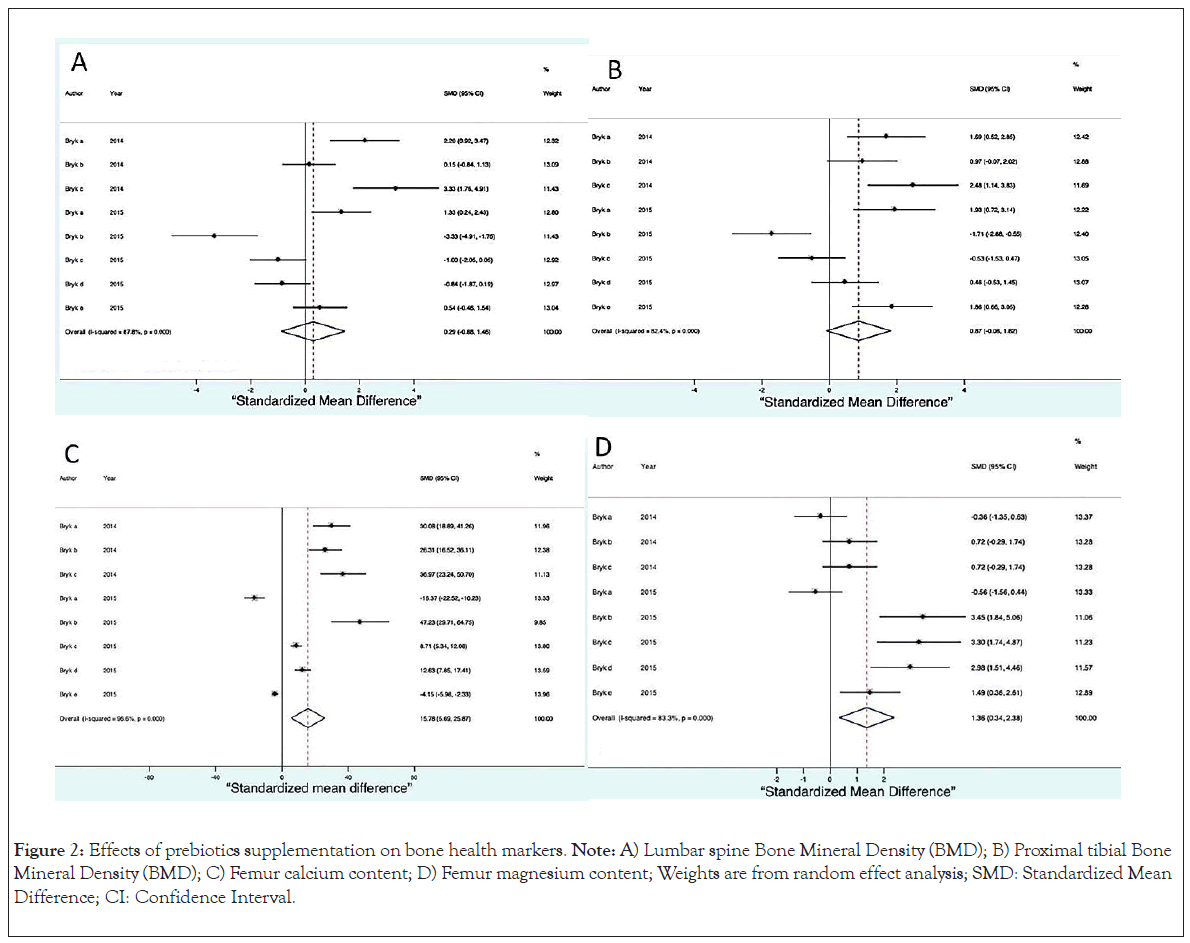
Figure 2: Effects of prebiotics supplementation on bone health markers. Note: A) Lumbar spine Bone Mineral Density (BMD); B) Proximal tibial Bone Mineral Density (BMD); C) Femur calcium content; D) Femur magnesium content; Weights are from random effect analysis; SMD: Standardized Mean Difference; CI: Confidence Interval.
The calcium content of the femur (SMD=15.78 95% CI, 5.69 to 25.87, p ≤ 0.0001) as shown in Figure 2C, was greater in the supplemented animals as was the magnesium content as shown in (SMD=136, 95% CI, 0.34 to 2.38, p ≤ 0.0001) (Figure 2D).
The serum concentration of cross-linked C-terminal Telopeptide of Type I Collagen (CTX) was lower in all supplemented groups (SMD=-6.74, 95% CI, -9.27 to -4.21, p ≤ 0.0001) as shown in (Figure 3A).
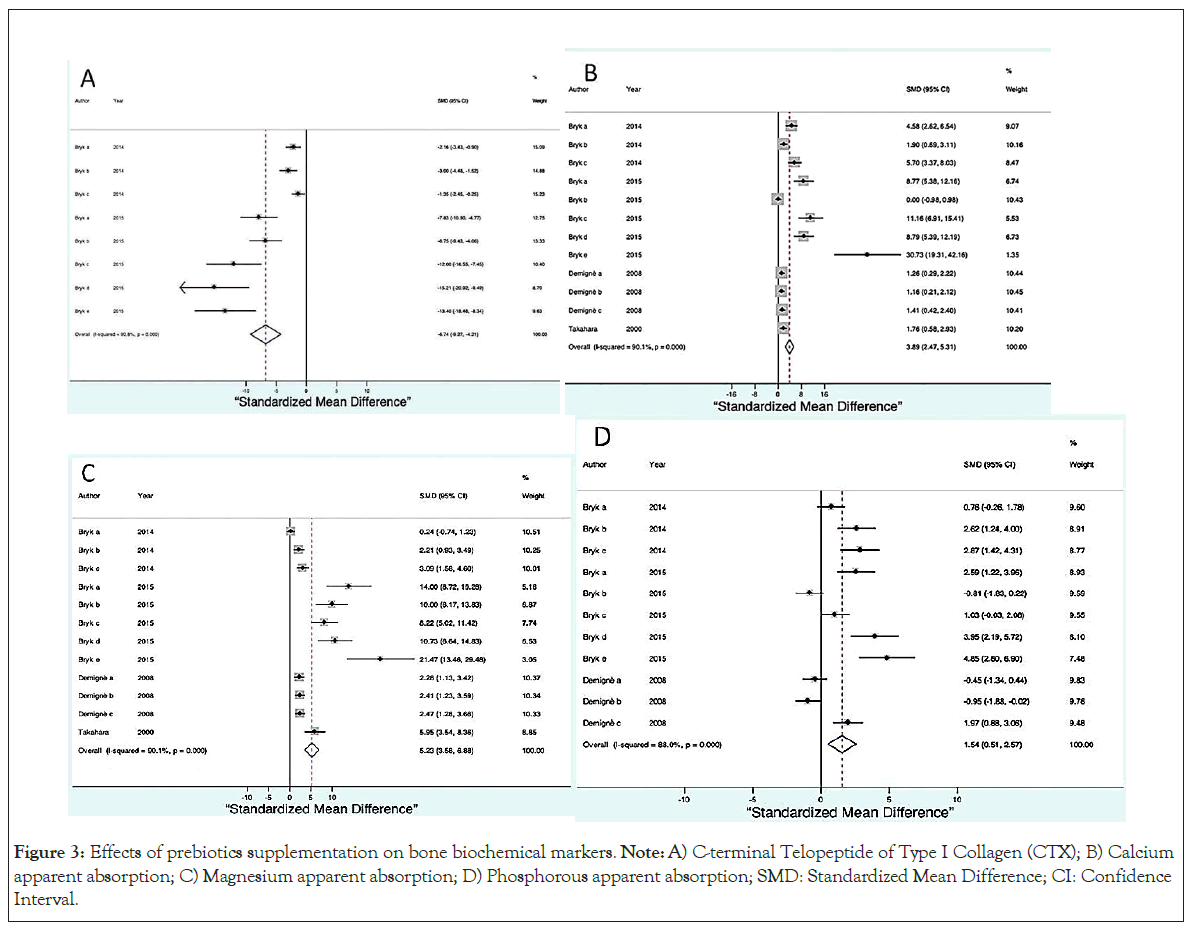
Figure 3: Effects of prebiotics supplementation on bone biochemical markers. Note: A) C-terminal Telopeptide of Type I Collagen (CTX); B) Calcium apparent absorption; C) Magnesium apparent absorption; D) Phosphorous apparent absorption; SMD: Standardized Mean Difference; CI: Confidence Interval.
Three variables indicated a greater absorption of nutrients in the groups supplemented with prebiotics: Calcium absorption (SMD=3.89, 95% CI, 2.47 to 5.31, p ≤ 0.0001) as shown in Figure 3B magnesium absorption (SMD=5.23, 95% CI, 3.58 to 6.88, p ≤ 0.0001) as shown in Figure 3C phosphorus absorption (SMD=1.54, 95% CI, 0.51 to 2.57, p ≤ 0.0001) as shown in Figure 3D.
In terms of secondary outcomes, we examined the pH of the cecum (SMD=-2.91, 95% CI, -3.58 to -2.08, p ≤ 0.0001) as shown in Figure 4A and observed that the pH was lower in all the supplemented groups, the total weight of the cecum was greater in the supplemented groups (SMD=3.88, 95% CI, 1.80 to 5.95, p ≤ 0.0001) as shown in Figure 4B and the Colony Forming Units (CFUs) in faeces (SMD=3.10, 95% CI, 1.19 to 5.00, p ≤ 0.0001) as shown in Figure 4C were more abundant in the supplemented groups.
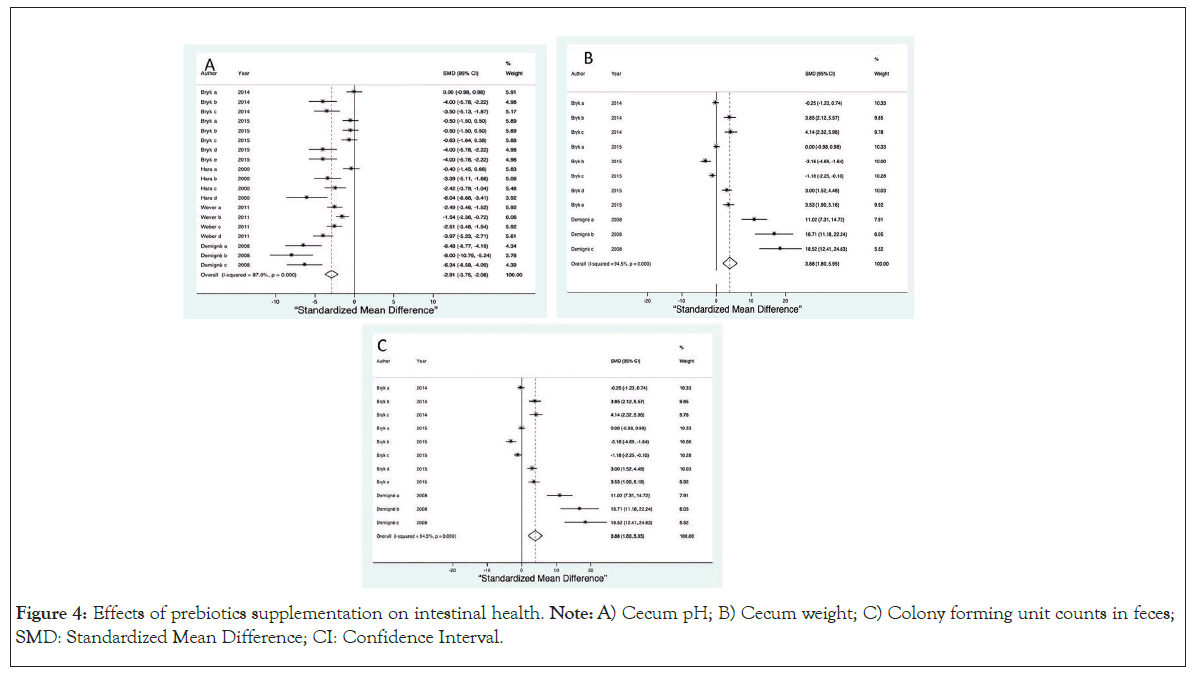
Figure 4: Effects of prebiotics supplementation on intestinal health. Note: A) Cecum pH; B) Cecum weight; C) Colony forming unit counts in feces; SMD: Standardized Mean Difference; CI: Confidence Interval.
The evidence of various isolated studies on lumbar spine BMD in mice showed improvement in BMD with prebiotic supplementation. The meta-analysis performed in the present study shows a global effect, supporting the evidence on the positive effects of this type of supplementation on BMD in all experimental groups, with either one prebiotic or combined prebiotics, regardless of the dose or combination and regardless of whether calcium was included in the supplement. The period of supplementation was maintained between 28 and 33 days in the studies where this outcome could be evaluated [14-16].
In the analyzed studies, an improvement in the BMD of the tibia was reported and the overall effect of the meta-analysis shows the positive effect of supplementation on tibial BMD. The Bryk et al., [14,15] and studies reported that this improvement was dependent on the amount of prebiotic contained in the diet. One of the important findings is about the calcium and magnesium content in the femur bone, which is higher in the supplemented groups than in the no supplemented groups. The Bryk et al., [14] study showed a lower impact on magnesium than on calcium since the confidence interval for some of the groups included the null value, but this may be due to the sample size of the groups. Compared to Bryk et al., [14,15] which reported a higher content of Mg in the tibias of the supplemented groups and the same trend but a minor effect for Ca, it should be considered that this study includes two healthy groups and three groups of animals recovering from malnutrition as those who responded better to supplementation and that the effect was observed not only in the BMD but also in the mineral content of bone.
Only two studies reported CTX, which is a marker of bone resorption that decreases in the presence of prebiotic supplementation [14,15]. In the Bryk et al., [15] study, groups that are recovering from malnutrition are included. In these groups, the decrease in CTX was greater since there was no removal of minerals from the bone, which supports the directionality of the calcium, magnesium and phosphorus absorption results that indicated a small but positive overall effect for all supplemented groups, despite that in some groups, the confidence interval included the null value. This phenomenon can be explained by factors such as the sample size in each group and the homeostasis itself, which involves the metabolism of these three elements. The techniques used to estimate the "apparent absorption" of these elements reported in the studies were evaluated over a period of 3 days, calculating the intake (diet) and evaluating the difference between the intake amount and the amount of these elements detected in the feces [14,15,17,18].
The pH of the cecum decreased in all the supplemented groups, showing a clear relationship, proportional to the prebiotic content. The greater the amount of prebiotic, the lower the pH of the cecum, thus the improved absorption of minerals in the intestine [14-16,19,20].
The CFU count in feces was greater in the supplemented groups as was the total weight of the cecum; these two variables are related since the weight is increased by the number of CFUs [14,15].
Although there were not enough menopause studies to conduct a meta-analysis, qualitative evidence indicated the same usefulness of prebiotics for improving BMD in studies conducted with the growth model.
Supplementation with prebiotics has positive effects on bone mineral metabolism, regardless of the amount or type of prebiotic, improving bone density and decreasing resorption; prebiotics are involved in various mechanisms, such as increasing CFUs, decreasing pH at the level of the intestinal cecum and increasing mineral absorption, so use is recommended in the population with mineral deficiencies as well as in the populations that are in periods of growth. More studies are required to corroborate the scope of long-term supplementation, as well as explore the studies of probiotics on bone mineral metabolism. Which is the prebiotic with the greatest potential for benefit.
The publication was financed with fiscal resources of the E022 program of the National Institute of Pediatrics. Luisa Díaz-García was supported by CONACYT, with the doctoral scholarship CVU98862.
The funding agency had no role in the study design, data collection or analysis, decision to publish or preparation of the manuscript.
DGL designed and coordinated the study, contributed to the data analysis and wrote the manuscript. ARH Contributed to designed the study, critically reviewed and revised the manuscript. JTF contributed to collected data and the data analysis and interpretation statistical analysis. DGL and ARH contributed to the data analysis and interpretation statistical analysis. All authors read, reviewed and approved the final version of the manuscript.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Diaz-Garcia L, Avila-Rosas H, Jimenez-Trejo F (2023) Impact of Prebiotics on Bone Mineral Metabolism in Murine Models: A Systematic Review. J Prob Health.12:355.
Received: 28-Dec-2023, Manuscript No. JPH-23-28780; Editor assigned: 01-Jan-0204, Pre QC No. JPH-23-28780 (PQ); Reviewed: 15-Jan-2024, QC No. JPH-23-28780; Revised: 22-Jan-2024, Manuscript No. JPH-23-28780 (R); Published: 28-Jun-2024 , DOI: 10.35248/2329-8901.24.12.355
Copyright: © 2023 Diaz-Garcia L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.