Immunome Research
Open Access
ISSN: 1745-7580
ISSN: 1745-7580
Review Article - (2024)Volume 20, Issue 2
The purpose of this review is to update the key findings from the scientific literature that provide explanations for many of the reported and analyzed adverse effects associated with the spike-based COVID-19 vaccination.
An overwhelming body of evidence supports the main mode of action of spike-based COVID-19 vaccines, namely the downregulation of ACE2 by spikes. Direct spike effects, synergisms and RAAS-independent responses complement and multiply the already deleterious effects on tolerability.
It has been repeatedly confirmed that the SARS-CoV spike protein alone is not only able to downregulate ACE2, but also to induce cell fusion, activation of TLR4, of co-receptors and gastrointestinal responses. The systemic and long-lasting detection of spikes after vaccination disproves the claimed regionally limited and short-lasting spike production and efficacy.
The exceptionally broad spectrum, frequency and severity of the reported ADRs associated with spike-based COVID-19 vaccination exceed the known level of conventional vaccinations.
According to ADR analyses, the spike-based vaccines possess an unacceptable class-specific, unique ADR/side effect profile.
From a pharmacological point of view, spikes are highly active substances, but not harmless antigens. For this reason, they are not appropriate for preventive immunization to avoid comparatively harmless infections.
Spike-based COVID-19 vaccination; Mode of action of vaccine spikes; Adverse drug reactions; ACE2 downregulation
Highest safety requirements must be placed on vaccines used for preventive health protection. The safety profile of COVID-19 vaccines should be particularly favorable, as the disease is only mild to moderate in the vast majority of cases and the Infection Fatality Rate (IFR) has been calculated to be quite low at 0.27%- 0.36% [1,2]. A representative mathematical analysis came to similar findings. The median IFR was 0.466% in April 2020 and fell by around 33% within 8 months to 0.314% (1 January 2021) before the vaccination campaigns began, with results varying greatly depending on age and country [3].
However, since the start of the COVID-19 vaccination campaign, spontaneous reports of suspected Adverse Drug Reactions (ADRs) and associated deaths have been accumulating at an unusually high rate, showing a broad spectrum of organ disorders analogous to COVID-19. Approximately 2.3%-2.6% of those affected by ADRs died from them [4]. The similarity of the systemic symptoms of COVID-19 disease in non-respiratory organs with the spectrum of ADRs of the vaccination suggested a common cause. As such, the binding of the spike subunit S1 of the SARS-CoV-2 viruses as well as the vaccine spikes to their host receptor enzyme ACE2 has been identified as the most important causative factor, providing COVID-19 disease an entirely new dimension among viral respiratory diseases and spike-based vaccines a class-specific, unique ADR profile.
Knowledge about the effectiveness of spikes has multiplied in recent years. Direct spike effects, synergisms and RAAS- independent responses complement and amplify the already known deleterious consequences of ACE2 downregulation. This review updates the most important findings from the scientific literature, which provide explanations for many of the reported and analyzed adverse effects [4,5]. Adverse reactions to other essential components of the finished vaccines, such as encoding mRNA, proinflammatory nano lipids, adenoviral vectors, as well as galenically necessary admixtures, such as adjuvants and excipients, and those caused by segregation or impurities, can contribute substantially to the ADR spectrum, e.g. through synergistic efficacy, but are not the subject of these considerations and must be considered separately.
The specific spike glycoprotein selected as the antigen for the development of spike-based COVID-19 vaccines (spike-inducing and protein-based vaccines) exerts multiple functions. It is responsible for inducing the organism’s immune response, TLR4- caused reactions, ACE2-receptor-mediated viral cell entry and spike-induced adverse reactions as well as RAAS-independent amino acid absorption in the gut. In addition, spikes can initiate ACE2-dependent cell fusion, but also ACE2-independent reactions, e.g. those of co-receptors (Figure 1).
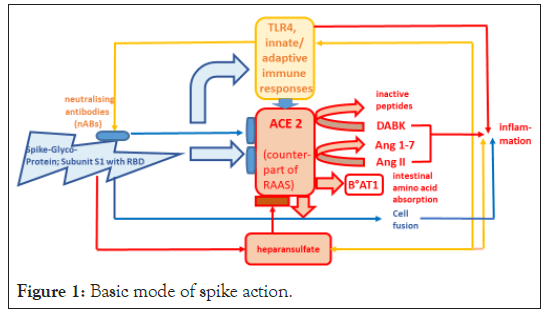
Figure 1: Basic mode of spike action.
As an antigen, the spike RBD of the S1 subunit triggers the cellular innate and adaptive immune response including the production of neutralizing antibodies (nABs), and the TLR4 activation. The binding of the S1 spike subunit via its RBD (non-neutralized) to the receptor enzyme ACE2 leads to its downregulation and impairment of its most important physiological function: the degradation of the pathophysiologically harmful angiotensin II (Ang II) to the mainly protective angiotensin 1-7 (Ang 1-7). In addition, the degradation of des-arg9-bradykinin (DABK) is inhibited. Ang II, DABK and TLR4 act synergistically to stimulate inflammation. The binding of S1 to the co-receptor Heparan Sulphate (HS) stabilizes the interaction with ACE2. HS moreover interacts with the immune system and has a pro-inflammatory effect. Spikes impair the co-operation of ACE2 with the amino acid transporter B°AT1 with regard to amino acid uptake in the intestine. In addition, spike proteins can fuse cells equipped with the ACE2 receptor to form syncytia. Cell fusion contributes to tissue inflammation.
Among several unique properties of the spike-inducing COVID-19 vaccines that could have implications for tolerability, the first to highlight is that, unlike conventional vaccines, no well-dosed and sufficiently tested antigen is used, but only a synthetic mRNA with the genetic code for the production of one of several antigens of the SARS-CoV-2 virus. The viral full-length spike was selected as the most promising antigen for the development of a vaccine; however, the recipient must produce this vaccine antigen himself. So far, there is no systematically established knowledge about the extent of spike antigen production, its duration, the influence of Endoplasmic Reticulum (ER) activity and other essential factors, about intracellular antigen transport, the detachment of the spikes from the cell membrane, and the proportion of non- neutralized spikes.
Because the systemic spike distribution is the precondition for interactions with receptors in various organs, such as the membrane-bound and soluble ACE2, toll like receptors (e.g. TLR4) or co-receptors, its detection is of eminent importance. Recently, some findings have shown a significant systemic distribution of spikes. The production of S1 antigens was already detectable on the first day after the first vaccination and extends beyond the injection site and the associated regional lymph nodes [6-8]. This is consistent with objectively detectable vaccination effects in asymptomatic vaccinated individuals (FDG uptake) up to 180 days after the second dose [9]. Spike protein was detected in the plasma of 96% of people shortly after vaccination, in 63% of vaccinated individuals one week after the first dose and spike antigen and vaccine mRNA were present in the germinal centers of lymph nodes up to 8 weeks after vaccination [10]. The systemic spike detection after vaccination disproves the claimed regionally limited spike production and efficacy.
ACE2 dependent spike reactions
Since the outbreak of SARS-CoV infections, it has been demonstrated that SARS-Co-viruses with their spike subunit S1 must initially attach to their receptor, Angiotensin-Converting- Enzyme 2 (ACE2) of the host cell membrane, before they can enter the cell compartment and replicate. The high-affinity interaction between SARS-CoV spikes and ACE2 is not only essential for virus entry into host cells, but also for mediating spike-triggered adverse reactions of vaccination via downregulation of ACE2.
It is important to note that since about 2005/2006 it has been repeatedly confirmed that the SARS-CoV spike protein alone is able to downregulate ACE2 in vitro and in vivo in the absence of other viral components [8,11-14]. The consequence is an activation of the RAAS with reduced Ang-II degradation, increase in Ang-II concentrations and impairment of the counter- regulatory, protective Ang-1-7/AT2R/MAS axis. AT1R blockade attenuates the effects of increased Ang II concentrations. In 2020, it was demonstrated [15] that spike protein (S1 subunit) in human lung epithelial cells effectively inhibits ACE2 expression, induces increased Ang-II levels and initiates the signaling cascade mediated by significantly increased AT1R expression, including induction of ADAM17 and inflammatory markers (IL-6 and other cytokines). Lei, et al [16] later described damage to vascular endothelial cells in animal experiments by ACE2 downregulation, impaired NO bioavailability and inhibition of mitochondrial functions in response to spike proteins. Furthermore, it could be confirmed that the S1 subunit of the spike glycoprotein is capable of causing systemic micro-endothelial cell damage. S1 subunit was only detected in cells with strong ACE2 expression (endothelia in micro vessels of the skin, subcutaneous fat, brain and liver); spike and ACE2 are strongly co-localized [17].
Even before the authorization of the first mRNA vaccines, it was known that SARS-CoV-2 and its spike protein directly enhance platelet aggregation and thus also thrombus formation through interaction with ACE2 [18].
A functional deficiency or lack of ACE2 not only favors the local accumulation of Ang II, but could also trigger a dysregulation of the closely linked bradykinin system, leading to an increase in des-arg9-bradykinin (DABK, Figure.1). The resulting synergism between Ang II and DABK, which can lead to hyperactivation of the immune system, provides a plausible explanation for a variety of adverse spike-related reactions, in particular for the dangerousness of the cytokine storm observed in rare cases of COVID-19 [19].
Another deleterious attribute of SARS-CoV-2 spike proteins is their ability to fuse cells and form syncytia [20,21]. Recently, the SARS-CoV-2 spike protein has been described as “enormously fusion active” [22,23]. In 2021, cell fusion was recognized as a trigger of the blood coagulation cascade. Viral fusogens are able to form large syncytia that tend to die, expose the thrombogenic basement membrane when detached and support platelet- dependent coagulation in this way. Syncytia formation can occur between infected, virus-replicating cells and healthy neighbouring cells, but also through extracellular spike-vesicles that connect healthy cells like bridges (“fusion from the outside”). This would explain cases of thrombosis also in non-infected tissue.
Besides, cell fusion may contribute to an excessive inflammatory response, tissue damage or production of cytokines to SARS- Co viruses or their components. For example, the cell fusion of pneumocytes induced by the SARS-CoV-2 spike (S) protein is accompanied by formation of microtubuli and a type 1 interferon response [24]. The spike receptor enzyme ACE2 is required for this process, because pathological cell fusion presupposes its existence.
Based on these findings, it can be assumed that the spikes formed after vaccination can lead to pathogenic cell fusion and may cause harmful effects similar to those of acute COVID-19 disease. Pathologically altered blood coagulation and endothelial damage after vaccination are class-specific adverse effects of spike-based vaccines.
Spike interactions with ACE2 co-receptors
Spike protein interactions with cells that have no detectable or low ACE2 activity suggest the presence of additional receptors. Heparan Sulfate (HS) is ubiquitously expressed on the surface of almost all mammalian cells, in the extracellular matrix and basement membrane. The structure of HS is highly variable, differs in different tissues and may be relevant to chemokine binding [25]. HS plays several important roles in the immune system; it regulates the cell adhesion, the development of leukocytes, and their migration, activates the immune system and inflammatory processes. HS interacts with the innate immune system, with TLR4 and other TLRs; HS seems to be a major modulator of the complement system [25,26]. HS is used by many viruses as a cofactor for attachment to host cells [27]. For SARS- CoV-2 infections, for example, it has been shown that the S1 subunit of the spikes can bind with its RBD to Heparan Sulfate (HS) on the cell surface in the sense of a co-receptor function, thereby stabilising or enhancing the interaction with ACE2 [28].
As a component of the endothelial glycocalyx, heparan sulphate is also involved in the regulation of blood coagulation; it increases the activity of anticoagulation factors such as Anti-Thrombin (AT) and Heparin Cofactor II (HCII). But, after binding of HS by the spike-protein, HS could no longer interact with AT/HC II, resulting in a rapid coagulation reaction and suggesting a direct effect of the spike-protein on development of thrombosis. Heparin lost its anticoagulation-regulating ability in a spike- protein concentration-dependent manner [29]. Furthermore, a synergism may be assumed between procoagulatory Ang II effects and direct spike-induced thrombotic effects with dramatic consequences for blood coagulation.
However, neither the involvement of the co-receptor HS in the blood coagulation disorders after spike-based vaccination nor the obvious synergism between binding of spikes to HS and the spike-induced downregulation of ACE2 or increase in Ang II has been investigated so far, although the frequency of corresponding relevant adverse effects is worrying.
An ACE2-independent dysfunction of human cardiovascular pericytes was first demonstrated in 2021 exclusively by recombinant spike proteins and with the involvement of CD 147/basigin; surprisingly, but pro-inflammatory responses were not regulated by involvement of CD147 [30].
In contrast to the impressive body of evidence for the causal role of the spike/ACE2 interaction in triggering organ symptoms caused by SARS-CoV-2 infection or vaccination, there is much less evidence for the involvement of S protein co-receptors (basigin/CD147, neuropilin/NRP-1, Heparan sulfate, CD209 L-SIGN, CD209 DC-SIGN, GRP78,Receptor Tyrosine Kinase AXL, ASGR1, KREMEN 1) [31]. There is still a need for further research in this area.
Presumed synergism between spikes induced ACE2 downregulation/Ang II elevation and TLR4 activation
The Toll-Like Receptor 4 (TLR4) is an essential member of innate immune system, localized on the cell surface (main site) of immune cells and expressed in the heart, lungs, liver, vasculature, brain and kidney. It recognizes as Pattern Recognition Receptor (PRR) extracellular Pathogen-Associated Molecular Patterns (PAMPs) and is crucial for triggering innate immune responses, inflammation also in the absence of infection and antigen- specific adaptive immune responses. Due to their complex and interacting downstream signaling pathways, they are able to influence numerous organ and cell functions.
Preconditions for spike-TLR4 interactions are the systemic availability of spikes and evidence of specific binding. Both are given. The SARS-CoV-2 spike glycoprotein, which acts as a Pathogen-Associated Molecular Pattern (PAMP), can directly bind and activate TLR4 with high affinity, independent of ACE2/ TMPRSS2 [32,33], resulting in NF-kB-activation and induction of proinflammatory cytokine expression (MyD88-dependent signaling pathway).
This is very similar to inflammatory reactions caused by RAAS activation. Angiotensin II, as the main effector peptide of the RAAS, is not only involved in vasoconstrictive effects, but also in inflammatory processes that could directly induce the activation of NF-kB. Additionally, Ang II also directly stimulates molecules are proved to be engaged in proliferation, differentiation, fibrosis, and inflammatory processes. Vascular oxidative stress, the synthesis of ROS and activation of NADPH oxidase can increase the activation of NF-kB and thus the occurrence of inflammation and fibrosis and lead to the progression of organ damage, such as diabetic nephropathy, if Ang II activity persists [34].
Decreased expression of ACE2 associated with lung pathology and inflammatory injury resulted in abnormal activation of TLR4 in an experimental acute lung injury model [35]. TLR4 deficiency prevented Ang II-induced vascular remodeling without affecting blood pressure, abolished Ang II-induced vascular ROS, inhibited Ang II induced NADPH oxidase activity, and enhanced upregulation of anti-oxidative ecSOD [36]. Cardiomyocyte-specific TLR4 deletion attenuates angiotensin II-induced hypertension, reduced cardiac hypertrophy, fibrosis, and dysfunction as well as cardiac inflammation (TNF, IL-1beta, IL-6, MCP-1 RNA expression).Conversely, Ang II significantly increased TLR4 gene expression in hypertensive mice [37].
In recent years, inflammatory processes involving the innate immune system and the RAAS have been demonstrated to play an important role in the development of various chronic diseases such as hypertension [37,38], diabetic nephropathy [34], vascular remodeling and cardiovascular disease [36]. In addition, dysregulation of TLR4 signaling has been implicated in the development and/or progression of atherosclerosis, myocarditis, cancer, neuropsychiatric and neurodegenerative diseases [39].
As PAMPS, the S1 subunit could trigger neuroinflammatory effects in the CNS (in the hypothalamus, hippocampus and frontal cortex as well as gene expression of microglia/brain macrophage activation markers, astrocyte activation markers, inflammasomes and proinflammatory cytokines) and behavioral consequences in rats (reduced exploratory and social behavior - “behavioral sickness response”). S1 also activated TLR2 and TLR4 receptor signaling in vitro [40]. Due to the widespread ACE2 expression, its involvement in the reactions cannot be ruled out, with the exception of those of the microglia, which do not express ACE2.
Furthermore, TLR4 has been identified as key mediator in long- term, specific and reversible cognitive dysfunction, microgliosis and loss of synapses after a single experimental brain infusion with spikes [41].
The involvement of the same effector molecules in initiation and maintenance of inflammation, proliferation and fibrosis supports the synergism between TLR4 activation and increased Ang II/AT1R activity. In cases of overstimulated TLR4 potentiated by a dysregulated RAAS, the synergism can intensify to severe inflammatory consequences (e.g. cytokine storm) or even contribute to a fatal outcome.
Taken together, these findings support that spikes are playing a deleterious biological role.
Consequences of spike induced impairment of ACE2
Pathogenic SARS-Co viruses and non-neutralized vaccine spikes use the enzyme ACE2 as target receptor in competition with the natural ligands angiotensin I/II, whereby the protective function of this enzyme is lost and the gateway opens for disturbances of homeostatic regulatory systems and of defense and repair mechanisms, such as hyperinflammation, remodeling, thrombo- embolic or immunological disturbances. Therefore, ACE2 plays a key role in understanding organ impairment caused by SARS-CoV-2 infection and systemic complications of spike-based vaccines [5].
ACE2 is predominantly membrane-bound, a smaller part circulates in soluble form. The soluble ACE2 is biologically active and characterizes various disease stages that are associated with RAAS activation and worse prognosis [14].The enzyme is expressed in almost all vital organs and organ systems, but to varying degrees in an organ-specific manner. ACE2 expression seems to be higher in Asian than in white and African-American people [14]. In principle, its expression explains the organ tropism of SARS-CoV-2, which extends far beyond the respiratory tract. ACE2 is particularly strongly expressed in cardiovascular tissues, especially in pleotropic pericytes, which are highly concentrated in cardiac muscle tissue. ACE2 is also strongly expressed in the small intestine, vessels, testes, kidney, brain and thyroid gland [14, 42-45]. Limited ACE2 expression is observed in the respiratory system both on the protein and mRNA level [46]. Recently, it has been demonstrated that viral spike proteins also activate intracellular signals that reduce ACE2 mRNA [12]. Practically nothing is known, however, about the reversibility/ irreversibility, duration and extent of membrane-bound/ soluble ACE2 downregulation, particularly in combination with changes in intracellular ACE2 mRNA. However, there are initial experimental findings on the specificity and dose dependence of the ACE2 reduction triggered by spikes [12].
The fact is that people with initially low ACE2 concentrations are particularly at risk when additional inhibitory influences are present. For example, in addition to spike-neutralizing immunological reactions, it seems possible that antibodies, autoantibodies, anti-idiotype reactions and/or genetic conditions can impair the efficacy of the protective enzyme ACE2 [47]. The latest findings following vaccination with Comirnaty® (spike- based mRNA vaccine) and Sinovac® (inactivated virus) are highly interesting. Both significantly increased ACE2 autoantibody IgG in 9.5%-12.6% and 3.3%-14.3% of individuals, respectively, on day 56 after vaccination. The values slowly decreased within 12 months [48]. However, due to the small number of cases and the lack of relevant data collection, no association with adverse vaccination events could be established; but involvement of ACE2-IgG antibodies in myo-/pericarditis according to Comirnaty® (n=43) could be excluded.
Furthermore, specific antibodies against S1-RBD were shown to cross-react with ACE2, probably due to structural similarity [49].
Recently, a positive correlation between ACE2 expression and anti-tumor signatures was found in various tumor types. ACE2 may protect against cancer progression, possibly by inhibiting tumor angiogenesis [50]. Further, especially tumor-specific studies in patients are required.
The best known physiological role of ACE2 is that of a counter- regulator in an activated Renin-Angiotensin-Aldosterone System (RAAS) with mainly cardio- and tissue-protective effects because ACE2 degrades angiotensin I/II (Ang I/II) and converts it to Ang 1-9/1-7. But the catalytic efficiency for Ang II is 400 times higher than for Ang I. ACE2 is thus an important modulator in limiting the deleterious effects of increased Ang II concentrations.
The multifaceted, multipotent octapeptide angiotensin II (Ang II) is the unquestioned major effector in the RAAS. Ang II affects the function of almost all organs, including the heart, kidney, vascular system and brain [53]. It is well accepted that Ang II is involved in vasoconstriction, increase in blood pressure, hypertension and associated end-organ injuries; vasopressin release; noradrenaline increase (elevated release, reuptake-inhibition); disturbance of the electrical conductivity of the heart; inducing of tachycardia and/or arrhythmia; increased oxidative stress; inducing deficient NO bioavailability; aldosterone-caused increase of sodium and water reabsorption; endothelial dysfunction; ADAM 17 increase; promoting (pro)-inflammatory (NF-kB) and pro-coagulant processes; microthrombus inducing; pro-arteriosclerotic, pro- fibrotic effects; promoting of hypertrophic and proliferative reactions; cardiac remodeling; proteolysis of skeletal muscles; affecting glucose/cellular metabolism; severe COVID-19 courses; beta-amyloid increase; downregulation of survival genes.
It has been known for decades that the RAAS is closely associated with the bradykinin system. Bradykinin, which in principle is a vasodilator with an extremely short-term and localized efficacy, is degraded by kinase II=ACE and its bioactive pro-inflammatory metabolite Des-Arg9-Brady-Kinin (DABK) by ACE2 [51]. Spike- triggered ACE2 downregulation can contribute to a potentiation of inflammatory Ang II effects through this mode of action. In a representative number of COVID-19 patients, it could be shown that DABK production was increased, especially in overweight people; the levels of Ang 1-7, on the other hand, were low as expected [52]. However, the involvement of DABK in the side effects of spike-based vaccination has not yet been proven.
Ang II also impairs adaptive immunity, for example by activating macrophages, which leads to increased production of IL-6, TNFα and other inflammatory cytokines [5].
Increased Ang II levels are significantly associated with depression, anxiety, Hypothalamic-Pituitary-Adrenal (HPA) axis hyperactivity, neuroinflammation, enhanced plasma cytokine levels and nitro-oxidative stress/production of NO. AT1 receptor blockers have been shown to be superior to the neuroprotective properties of ACE inhibitors. Anxiolytic/antidepressant effects of RAAS blockers may be mediated by their anti-inflammatory and/or anti-oxidative stress effects [54].
Central and peripheral catecholaminergic activities amplify Ang II effects.
If inhibition of Ang II degradation occurs, then its harmful effects can establish themselves. Therefore, knowledge of Ang II effects is an indispensable requirement for understanding the adverse reactions associated with spike-based vaccines and possible therapeutic approaches [5].
The role of ACE2 in the gastrointestinal tract
The intestinal epithelium is equipped with all components of the RAAS. ACE2 is strongly expressed in the gut especially in colon [55,56]. Members of the gut microbiome significantly modulate gastrointestinal and pulmonary ACE2 expression. However, the mechanisms by which the microbiome regulates ACE2 expression are still unclear and require further clarification [57].
Faecal viral shedding outlasts the negative nasopharyngeal swab [58], and therefore the gut may serve as a viral reservoir and may be involved in the development of the so-called “long COVID”.
Docking of SARS-CoV-2 spikes to host cells is known to lead to downregulation of ACE2, followed by a pathophysiologically detrimental increase in Ang II levels, AT1R activation and increased intestinal barrier permeability [58]. However, in addition to its protective catalytic activity, ACE2 plays another important physiological role in the gastrointestinal tract. ACE2 is involved in the regulation of dietary amino acid homeostasis, intestinal inflammation, gut microbiota composition, innate immunity, and glucose absorption [58-60], all of which are affected by the efficacy of spikes. The significantly increased intestinal susceptibility to inflammation could be due to altered availability of amino acids in the gut, particularly by affecting local tryptophan homeostasis [60]. Therefore, it is plausible that a functional impairment or deletion of ACE2 could lead to a reduced uptake of L-tryptophan [56,61].
Tryptophan as a precursor of serotonin is of great importance for central nervous transmission processes, which are disturbed in depression, for example. Decreased serotonin production may result in mood disturbances. Between Ang II and serotonin exist an interaction; Ang II regulates stress-related effects by modulating serotonin synthesis and release [54]. An involvement of low circulating serotonin levels in Long-COVID is discussed [62].
In addition to membrane-bound ACE2, the amino acid transporter BoAT1, whose expression on the luminal surface of intestinal epithelial cells is stimulated by ACE2, is required for the sodium-dependent uptake of neutral amino acids. Structural analysis revealed that the S1-RBD components of two spike protein trimers interact with a complex of ACE2 and BoAT1 [63]. In the absence of intestinal BoAT1 protein expression due to lack of ACE2 enzyme, serum levels of the neutral amino acids valine, threonine and tyrosine as well as of the essential amino acid tryptophan were significantly reduced; the mTOR activity, which is involved in cell proliferation and protein synthesis and which is activated by dietary tryptophan, is also reduced [60,61].
Recently, it could be shown, that the gut microbiome reflects immunogenicity and was associated with vaccine related adverse events. Vaccinated individuals with a higher content of beneficial bacteria may have an optimal immune response and stronger protection. A higher content of P. copri and Megamonas species was associated with less adverse events. BNT162b2 vaccinees who reported any adverse reaction had a significant decrease in observed bacterial species richness [64].Further investigations are required to verify these initial results.Selected consequences of spike-based COVID-19 vaccination
The unusually wide range and frequency of reports of suspected adverse reactions and their fatal consequences, which have also increased in Europe since the conditional marketing authorization of COVID-19 vaccines, nevertheless represent only a small proportion of the actual occurrence. The informative value of the adverse drug reaction figures documented by the established pharmacovigilance systems is considerably impaired by underreporting due to a lack of knowledge and/or ignorance of a possible correlation, low willingness to report and provide information on the part of the healthcare providers or a focus on other necessities.
In this context, the growing total number of individual cases (n=2,256,506 cases in European countries up to 31 July 2023) with adverse effects and their fatal outcome (n=51,740) associated with spike-based COVID-19 vaccines is already very alarming. The upward trend (cumulative number of vaccinated persons with adverse events) is unbroken. With an average of 2,338 people affected per day in European countries by 31 July 2023, of whom an average of 54 per day died from adverse events (2.3% of those vaccinated with adverse events), decades of experience with conventional vaccines have been far exceeded [4].
In general, more women (approximately 69%) than men were affected by ADRs; the majority (about 76%-78%) were in the age range of 18-64 years [4,65].
Frequently observed organ-related ADRs after COVID-19 vaccination were such of the nervous system (between 16%-20% of all reported ADRs depending from vaccine), musculoskeletal system (between 11.7%-14.5%), gastrointestinal tract (between 7.5%-9.3%), and skin (between 3%-5.1%), but cardiovascular ADRs are among the most dangerous associated with COVID-19 vaccination (fatal outcomes due to vaccine related cardiac disorders: between 8,44%-14,51%). Michels et al [66] were able to demonstrate from the analysis of deceased patients that a cardiovascular risk was already recognizable at the time of emergency use authorization of BNT162b2, which became even more evident during a 6-month follow-up period. However, neither the vaccine manufacturer nor the regulatory authorities clarified this finding.
There is only limited information on the duration of vaccination sequelae. Using the diagnostic FDG-PET imaging procedure, it was possible to demonstrate that myocardial FDG uptake activity was significantly increased up to 180 days after the second mRNA vaccination in asymptomatic individuals (n=700) compared to non-vaccinated patients (n=393).This was also true for liver and spleen; axillary lymph node FDG uptake lasted for a maximum of 120 days. The increase in FDG uptake in myocardial and axillary tissue was also shown in an intra-individual comparison (n=16). The increase began on the first day and lasted for 30 days in axillary lymph nodes before falling to the control level by the 120th day; in heart tissue, the values remained relatively constant at an elevated level up to 180 days, starting from a higher initial level. No differences in myocardial FDG uptake were observed in vaccinated patients when stratified by age, sex, or vaccine type [9]. These findings demonstrate a quick systemic response and an organ damage potential due to spike-inducing mRNA vaccination that lasted on average at least up to 180 days after the second dose. Elevated autoantibodies against ACE2 after Comirnaty® vaccination could even be detected up to 12 months [48]. Further examinations involving other organs such as the brain, kidneys, vascular system etc. as well as measurement of corresponding tissue markers (CK, Troponin, BNP etc.) and RAAS-parameters (ACE2/Ang II-activity, auto-/antibodies etc.) are desirable.
Unsurprisingly, Ang II-mediated acute effects such as tachycardia, arrhythmia, atrial fibrillation/flatter, bradyarrhythmia and impaired pacing and conduction (n=57,438 combined; 4.7% of vaccinated individuals with ADRs [4]) dominated the cardiovascular ADR profile of Tozinameran, followed by reports of blood pressure increase (n=25,907; 2.1% of vaccinees with ADRs [4]). According to the latest analysis by Cocco (2023), cardiac arrhythmias/heart rhythm disorders are not uncommon after vaccination and were never significantly associated with vaccination other than the COVID-19 vaccines [67]. The detection of viral spikes in the atrioventricular node of the cardiac conduction system of a deceased patient with cardiac arrhythmia confirms the probability of a direct influencing of spikes on the conduction system of the heart [67,68].
The frequency of blood pressure elevations is alarming and signals a significant cardiovascular risk that should no longer be downplayed, especially as the connection between the restriction of the vasodilatory/vasoprotective effects of ACE2 and the Ang 1-7/AT2R/MasR axis due to the spike efficacy and the reactive increase in blood pressure can almost be characterised as classically pathophysiological.
The high number of cardiac arrests, sudden cardiac deaths and deaths associated with Tozinameran (n=5,424) characterises the severity of the cardiovascular burden. Interestingly, the proportion of those affected has halved between 5 June 2021 and 31 July 2023 [4,69]. The underlying causes (e.g. decline in sensitive individuals, focus on other ADRs, growing ignorance etc. ?) have not yet been investigated and remain unknown.
The fatal outcome may be the result of an acutely dysregulated Renin-Angiotensin-Aldosterone System, but may also be the consequence of exacerbation of pre-existing or developing myocardial dysfunction (heart failure, cardiomyopathy, cardiovascular collapse and ventricular fibrillation or flutter: n=16,778 combined) or myo-/pericarditis (n=23,775) [4]. A late event up to one year after vaccination is also conceivable due to the detection of ACE2 autoantibodies.
Evidence for the involvement of AngII/NA was provided by the histopathological clarification of the sudden death of two adolescents after the second BioNTech vaccination [70]. The investigations revealed a stress cardiomyopathy caused by catecholamines, which differed from the typical myocarditis findings. The authors considered epicardial vasospasm, microvascular dysfunction and direct toxic effects on the cardiomyocytes to be the cause of the catecholamine-induced myocardial injury [70]. No evidence of a hypersensitivity reaction was found. The fibrosis observed in one case is consistent with the pattern of RAAS-induced subacute sequelae.
In particular, the increasing number of unexpected sudden cardiac deaths among apparently healthy athletes is attracting attention [71]. However, neither this nor the plausible link between RAAS activation and sudden death have so far prompted the manufacturers or the regulatory authorities to take appropriate action.
Out of this spectrum, only myo-/pericarditis (23,775 reports; 5th in terms of frequency and 13th in terms of cardiovascular risk [4]) has so far been attributed a vaccine-related signaling effect by the EMA. The focus of attention on this consequence of vaccination has certainly contributed to the fact that reports have increased more than 8-fold within about 2 years in European countries. Although the course of myocarditis is usually characterized as mild, severe courses and fatalities have also been reported. Recently, it has been shown that myocarditis after vaccination is associated with normal adaptive and T-cell immunity, but modest innate inflammatory activation with increased cytokine levels, an increase in neutrophil granulocytes and a decreased platelet count. Furthermore, individuals who developed myocarditis had markedly higher levels of free full-length spike protein in circulation, unbound by anti-spike antibodies, than control subjects [72]. Consequently, cytotoxic and ACE2 downregulation- mediated spike effects cannot be prevented in the absence of antigen neutralization and can thus contribute to development of myocarditis.It is therefore extremely important to clarify the pathogenesis and consequences of high concentrations of non- neutralized spikes in the plasma of vaccinated patients with myocarditis.
It is obvious that Ang II-induced vasoconstriction, platelet activation and/or tachyarrhythmia can unavoidably result in ischemic sequelae. In the case of pre-existing arteriosclerotic vascular injury, this could have a particularly detrimental effect. This would explain the high number of reported cases of coronary heart disease and myocardial infarction (n=9,912) [4].
Other parts of the body may also be affected. For example, there are initial reports of a vasoconstrictive genesis of flash-like headaches/severe headache attacks associated with COVID- 19-disease or vaccinations, similar to reversible cerebral vasoconstriction syndrome (RCVS) provoked by vasoactive agents [65,73,74]. Furthermore, impaired blood supply to the vestibular organ can result in dizziness (6.4%-8.3% of vaccines with ADRs) and/or balance disturbances (0.38%-0.41%) [65]. Ischaemia and hypoxia are also known to be contributory to central nervous function deficits, such as transient memory loss or impairment (approximately 0.13%-0.25% of vaccinees with ADRs), impaired attention or consciousness (0.03%-0.46%), brain fog (5.9%) or blood-brain barrier dysfunction. The impairment of amino acid absorption in the gut can cause a deficiency of transmitters in the CNS and thus initiate the development of mood disorders or contribute to long-lasting behavioral changes.
Peripheral nerve dysfunctions such as paranesthesia and sensory disturbances were rather common (2.6%-3.4%, average 3.2% in vaccinated patients with ADR [65]) and may indicate also disturbed blood supply via the vasa nervorum to low or unmyelinated nerve fibers caused by vasoconstriction, vasculitis/ vasculopathy or inflammatory processes. The detection of autoantibodies against AT1R, ACE2 and/or the Mas-receptor in neuropathy supports the causal involvement of a dysfunctional RAAS.
The involvement of endothelial, vasoconstrictor or direct spike-induced disturbances of skeletal musculature (for example myalgia, muscle spasms, atrophy, proteolysis, and rhabdomyolysis) and skin florescences is discussed [65].
Very early after the start of the COVID-19 vaccination campaign (December 2020 to February 2021), it was noticed that Immune- Mediated Diseases (IMD), particularly autoimmune rheumatic in nature, occurred in temporal association with the 1st or 2nd dose of vaccination [75]. This was followed by several reports of cases of polymyalgia rheumatica as well as cases of autoimmunological caused neurological diseases by independent authors without considering the involvement of impaired RAAS/Ang II homeostasis [5]. Since Luft et al., [76] at the latest, it can be considered proven that the impairment of immune mechanisms contributes to end-organ damage caused by Ang II.
The deleterious spike protein-induced fusion of cells and the formation of syncytia could explain the unusual frequency of pathological pulmonary symptoms after the application of mRNA vaccines as well as their thrombogenic potential and enhance the procoagulant effects of Ang II and the direct dose- dependent spike activation of platelets [18]. The high rate of thrombo-embolic complications (in the 2-4 digit range within the first 5 months since the start of the vaccination campaign) following spike-based vaccination is therefore not surprising [5].
Fusion of neurons or fusion between neurons and glial cells can also occur in the central nervous system, which can be involved in the triggering of neurological symptoms, e.g. persistent neuropathic pain [20].
Gastrointestinal symptomatology (e.g. diarrhea, nausea, vomiting, abdominal pain, anorexia) is very common in COVID-19 disease (12%-61% of those affected, [77]) as well as after vaccination (7.5%-9.3% of individuals with ADRs) [65].Microbiota-targeted interventions have the potential to modulate the efficacy of COVID-19 vaccines, as an initial study suggested. Side effects, including gastrointestinal, were significantly more frequent after the 1st and 2nd application of the mRNA vaccine BNT162b2 (93%-95%) than after the inactivated vaccine CoronaVac (62.2%- 67.6%) [64].
The vascular system, which is equipped with all components of the RAAS, must be seen as a link between pathophysiological biochemical processes and symptomatic tissue damage.
In the majority of cases, the analyzed acute to subacute adverse effects of spike-based vaccinations convincingly reflect the mode of action of the spikes-ACE2 downregulation/Ang II increase and impairment of the protective, anti-inflammatory Ang 1-7/AT2R/ MAS axis. An impressive volume of recent findings supports this statement.
It is evident that the multiple functions of the RBD of the S1 subunit of the spikes (triggering innate and adaptive immunity, neutralizing antibody production, TLR4 activation, high affinity interaction with ACE2/competition with Ang II, cell fusion, direct co-receptor effects) and those of ACE2 (Ang II degradation/RAAS counter-regulator, SARS-CoV-2 viral receptor, vaccine spike receptor, cell fusion mediator, DABK-degradation, RAAS- independent responses) significantly influence the extent of adverse spike efficacy.
Adverse spike reactions involving the ACE2 receptor, which are not induced by activation of the RAAS but by influencing intestinal amino acid absorption, require special attention, also due to possible long-term and long-distance effects (insufficient supply of transmitter precursors, followed by CNS dysfunctions).
When triggering inflammatory processes, the synergism between Ang II, TLR4 and DABK as well as with the co-receptor Heparan Sulphate (HS) and the consequences of spike-induced cell fusion must be taken into account. In addition to direct spike-induced platelet aggregation, the co-receptor Heparan sulphate, cell fusion and procoagulatory Ang II effects possess a dangerous synergistic thrombo-embolic potential. Furthermore, some of the spike- induced Ang II effects may be catecholaminergically enhanced.
These factors basically determine the harmful consequences of spikes and substantiate the qualitatively identical, class-specific ADR profile of spike-based COVID-19 vaccines.
According to recent findings, the systemic spike distribution after vaccination disproves the claimed regionally limited spike production and efficacy; effects (e.g. autoantibodies against ACE2) of spike-based vaccinations could be detected for up to one year.
A persistent and possibly self-perpetuating functional impairment of these regulatory systems could contribute to harmful long-term consequences that are still largely unknown and unexplored.
From a pharmacological point of view, spikes are highly active substances, but not tolerable simple antigens in the human organism. For this reason, they are not suitable for preventive immunization to avoid comparatively harmless infections.
For constructive input I am grateful to Mr. Em. o. Univ. Professsor Dr. med. Hartmut Glossmann, Inst. f. Biochemical Pharmacology of the Medical University of Innsbruck.
None to report
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Not required
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar].
[Crossref] [Google Scholar].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref][Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref][Google Scholar][PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar].
[Crossref] [Google Scholar] [PubMed].
[Crossref][Google Scholar] [PubMed].
[Crossref][Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar].
[Crossref] [Google Scholar].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
Citation: Lehmann KJ (2024) Impact of SARS-CoV-2 Spikes on Safety of Spike-Based COVID-19 Vaccinations. Immunome Res. 20:267.
Received: 10-May-2024, Manuscript No. IMR-24-31313; Editor assigned: 13-May-2024, Pre QC No. IMR-24-31313 (PQ); Reviewed: 27-May-2024, QC No. IMR-24-31313; Revised: 04-Jun-2024, Manuscript No. IMR-24-31313 (R); Published: 11-Jun-2024 , DOI: 10.35248/1745-7580.24.20.267
Copyright: © 2024 Lehmann KJ. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.