Journal of Cancer Science and Research
Open Access
ISSN: 2576-1447
ISSN: 2576-1447
Research Article - (2023)Volume 8, Issue 1
BACKGROUND: Patients with metastatic gastric adenocarcinoma have a relatively poor prognosis with a median survival of 6 months. The three-drug regimen of docetaxel, oxaliplatin and fluorouracil (DOF) has been shown to improve survival compared to the two-drug regimen of docetaxel and oxaliplatin with similar toxicity. However, there is no published Indian experience with this regimen.
AIM: The aim of this study was to evaluate the efficacy of DOF regimen in Indian patients.
MATERIAL AND METHODS: All patients with metastatic gastric cancer who were treated with DOF regimen at our tertiary care centre in North India from 2014 to 2018 were retrospectively reviewed. 15 patients received DOF regimen as docetaxel 60 mg/m2 and oxaliplatin 85 mg/m2 on Day 1, and continuous infusion 5-FU 750 mg/m2 over 48 hours with pegylated-granulocyte colony-stimulating factor support every 2 weeks until progression or unacceptable toxicity. The endpoints were overall response rate (ORR), progression-free survival (PFS) and overall survival (OS) which were evaluated using Kaplan-Meier analysis.
RESULTS: Fifteen patients with a median age of 52 years were identified; 73% were males. Overall response rate was seen in 87% patients (complete response: 20%, partial response: 60%, stable disease: 7%) and progressive disease in 13%. With a median follow-up of 14 months, the median PFS was seven months and the median OS was 16 months from the start of therapy. 1-year PFS was 22%, and 1-and 2-year survival was 79% and 26%, respectively.
CONCLUSION: DOF regimen is an effective and feasible regimen in patients with metastatic gastric cancer and shows similar survival to historical regimens.
Advanced gastric carcinoma; gastric cancer; DOF regimen; progression-free survival; overall survival; disease free survival.
Gastric cancer is among the most common causes of cancer-related deaths throughout the world and ranked as the third and fifth causes of deaths due to cancer in men and women, respectively. The highest rate of gastric cancer has been reported in Eastern Europe, Eastern Asia, and South America.[1],[2] The overall survival (OS) remains poor in cases of advanced gastric cancer; the median OS has been reported as approximately 7.5–12 months vs 3–5 months among patients receiving chemotherapy compared with the patients considering best supportive care alone. Though the results have not been very optimistic, systemic chemotherapy is associated with substantially superior survival and a high quality of life compared with supportive care alone.[3],[4], [5]In India, gastric cancer constitutes a major health disease burden and is the most common cause of cancer deaths. [3],[6] Although there are advancements in the treatment of gastric cancer worldwide, the advanced disease has a relatively poorer prognosis with a 5-year OS of 14%.[3] Introduction of combination chemotherapeutic regimens including 5-fluorouracil (5-FU) and platinum have had a survival advantage over best supportive care.[4] Although optimistic responses have not been proved, a superior OS and better quality of life have been observed with systemic chemotherapy compared to supportive care. Though docetaxel, cisplatin, and infused 5-FU (DCF) has shown to be more efficacious than cisplatin and infused 5-FU regimen (CF), grade 3–4 treatment-emergent adverse events have been shown comparatively higher with DCF. [7] The follow-up reports showed a better quality of life and clinical benefit with DCF regimen.[8],[9] But still, there is a requirement to find effective regimens with reduced toxicity. In combination protocols for advanced gastric cancer, oxaliplatin has shown higher progression-free survival (PFS) rate than cisplatin with significantly lower incidences of most of the adverse events. In the elderly subset of patients, oxaliplatin showed superior response rate, time to treatment failure, PFS, and better tolerability than cisplatin.[10] This combination of 5-FU and oxaliplatin is studied in several phase II studies with different doses, schedules, with all having outcomes that were significant and satisfactory.[11],[12] Docetaxel, oxaliplatin and capecitabine (DOX) regimen has been proved as superior alternative regimen for first-line treatment of advanced gastric cancer compared to docetaxel, oxaliplatin and 5-FU (DOF) and paclitaxel, docetaxel and 5-FU (PDF) regimens.
[13] DOF regimen has been shown higher overall response rates (ORRs), disease control rate (DCR), PFS and OS as compared to 5-FU, leucovorin, and oxaliplatin regimen (FOLFOX) with no significant difference in toxicity.[14] However, DOF regimen is no published Indian experience with this regimen. This retrospective study was conducted to evaluate the efficacy of DOF regimen in Indian patients.
All patients with metastatic gastric cancer who underwent treatment at our tertiary care centre in North India from Year 2014 to Year 2018 were reviewed retrospectively. The patients were included if they met all of the following inclusion criteria:
1) had age between 18 and 75 years at diagnosis; 2) had histology proven gastric cancer; 3) had metastatic disease 4) received treatment with DOF regimen for ≥ four weeks. The patients who had received DOF for less than four weeks were not included in this retrospective analysis, hence including 15 patients in this study. All of these patients received DOF regimen as docetaxel at a dose of 60 mg/m2 and oxaliplatin at a dose of 85 mg/m2 on Day 1 and continuous infusion of 5-FU at a dose of 750 mg/ m2 over 48 hours with pegylated-granulocyte colony-stimulating factor support every 2 weeks until progression or unacceptable toxicity. Information regarding demographic characteristics, prior treatment, and adverse events was retrieved from the available data. The endpoints were ORR, PFS and OS. Response was assessed after fourth cycle and then after every four to six cycles. Therapy was given until progression or unacceptable toxicity.
IBM SPSS statistics version 20 (IBM corporation, United States of America) was used for the statistical analyses. Clinical benefit rate was defined as the percent of patients without clinical or radiological progression at three months. Overall survival was defined as a period from the date of start of chemotherapy to the date of death from any cause. Progression-free survival was defined as the time period from the date of start of chemotherapy to the date of radiological/clinical progression of disease or death due to any cause. Kaplan–Meier analysis was performed to evaluate OS and PFS. Categorical variables were compared using the Chi- square test or Fisher’s exact test when indicated. Predictive factors for OS or PFS were analyzed through Cox regression analysis. All analyses were censored on 01 September 2018.
Baseline characteristics
Records of 17 patients who met the inclusion criteria were retrieved. From this cohort, two patients violated the inclusion criteria as they had received DOF therapy for less than four weeks; therefore, they were excluded from the study. The remaining 15 patients constituted the study group. The baseline characteristics of these 15 patients are described in Table 1. The median age was 52 years (range: 26 to 63). A majority of patients were male 10 (73%). Six (40%) patients had Eastern Cooperative Oncology Group (ECOG) performance status (PS) between 0-1, five patients (33%) had ECOG PS 2, two patients (13%) had a PS 3 and two patients (13%) had PS 4. Of the two patients who had received prior chemotherapy, one patient had received capecitabine, and one patient had received capecitabine and oxaliplatin.
| N=15 | |
|---|---|
| Median age, year | 52 |
| Minimum | 26 |
| Maximum | 63 |
| Gender | |
| Male | 10 (73%) |
| Female | 5 (33%) |
| ECOG PS, n (%) | |
| 0-1 | 6 (40%) |
| 2 | 5 (33%) |
| 3 | 2 (13%) |
| 4 | 2 (13%) |
Table 1: Baseline Characteristics of Included Patients
Among the 15 patients evaluable for response, three (20%) had complete response, nine (60%) had partial response, one (7%) patient had stabilized disease, and remaining two (13%) had progressive disease (Table 2).
| DOF N=15 | |
|---|---|
| CR | 3 (20%) |
| PR | 9 (60%) |
| SD | 1 (1%) |
| PD | 2 (13%) |
Table 2: Tumor Response to Treatment
The median follow-up of the cohort was 14 months. The median PFS was seven months and the median OS was 16 months from the start of therapy (Figure 1 and Figure 2). 1-year PFS was 22%, and 1-and 2-year survival was 79% and 26%, respectively.
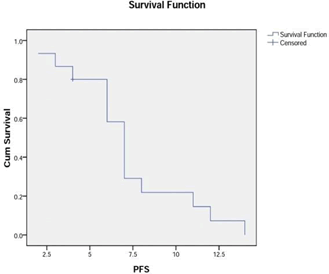
Figure 1: Progression-free survival using Kaplan–Meier analysis
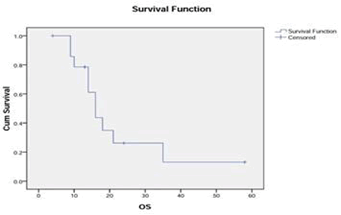
Figure 2: Tumor Response to Treatment
Gastric cancer constitutes one of the most common malignancies in India; 40% of the patients are diagnosed at the advanced stage in spite of the decline rates of incidence and mortality over the last two decades.[3] The prognosis for advanced gastric cancer is not satisfactory with a 5-year survival rate of <10% and median OS <1 year.[15] For patients with metastatic gastric cancer, not suitable for surgery or developed postoperative extensive metastasis, chemotherapy remains the standard of care. The research now on advanced gastric cancer has been focusing on finding better combination regimens that shall improve the outcomes with the least possible toxicities. This study was conducted to evaluate the efficacy of DOF regimen in Indian
patients; DOF regimen data have been published only for other countries’ patients before this.Docetaxel has been proved as an efficacious agent, and in TAX325 study, it has been dictated as a novel option for treating advanced-stage gastric cancer with significant efficacy.[16] DCF combination regimen vs CF regimen in the V325 trial, a randomized multinational phase II/III trial of untreated advanced gastric cancer patients, gave a median time to progression of 2.2 months that was significantly longer than the control arm (p <0.001) and OS advantage of two months, with 1-year survival of 40% vs 32%. Though DCF has shown to be more efficacious than CF, grade 3 and 4 treatment-emergent adverse events have been shown comparatively higher with DCF. [7] A phase II study of 5-FU, leucovorin, oxaliplatin and docetaxel regimen reported an improved median PFS of nine months and OS (median) of 17.3 months in older adults with locally advanced or metastatic oesophagogastric cancer.[17] One more Phase II study in advanced gastric cancer patients treated with DOF regimen reported the median OS of >14 months and the duration was substantially higher than to 8-9 months of OS were reported in previous international multicenter studies.[18] In the combination protocols for advanced gastric cancer, oxaliplatin (+leucovorin and 5-FU) have shown significantly higher 6-month PFS rate than cisplatin (leucovorin + 5-FU; p = 0.024) with significantly lower incidences of most of the adverse events. In the elderly subset of patients, oxaliplatin showed significantly superior response rate, time to treatment failure, PFS, and better tolerability than cisplatin.[10] To our knowledge, though some studies have been conducted on DOF regimen before the present study, these were conducted in the countries outside India. It is not known whether the DOF regimen is suitable for Indian patients or not. This was the reason to perform this study. This study was conducted in 15 patients with the aim of evaluating the efficacy of DOF regimen in Indian patients with metastatic gastric adenocarcinoma. Among the 15 patients evaluable for response, 20% had complete response, 60% had partial response, 1% had stabilized disease, and remaining 13% had progressive disease. The corresponding values in a previous study were 5%, 45%, 41%, and 9% in which the number of patients was 58 in the DOF group.[14] The variation in the rate of incidences seems due to the small number of patients in the present study. A study of 97 consecutive advanced gastric cancer patient compared DCF (n=53) and three modified regimens of DOX (n=14), DOF (n=13), and PDF (n=17). The study reveals no statistical difference among the different treatment groups.[13] Also, a study comparing DOF and FOLFOX in 118 randomised patients showed higher ORRs, DCR, PFS and OS for DOF as compared to FOLFOX with no significant difference in toxicity. The median OS and PFS in DOF group were significantly higher than in the FOLFOX group (16.3 vs 11.2 months and 8.2 vs 6.4 months; p<0.001) with acceptable toxicities in both groups,[14] The results in the DOF group are consistent with the results observed in the present study where medianOS was 16 months and PFS was seven months. Another retrospective analysis of 88 patients comparing DOF (n=45) and
ECF (epirubicin, cisplatin, 5-FU) regimen (n=43) showed higher overall response rate (42.2% vs 37.3%), tumour control rate (80% vs 60.6%), median PFS (6.7 vs 5•0 months) and OS (11.4 and 9.8) in the DOF group than in the ECF group.[19]In the above review analysis of all the studies, DOF regimen appears to be an efficacious and safe therapy for advanced/metastatic gastric carcinomas. All these studies have been performed in the western population and no Indian data are yet available to conclude the same. It is well known that patients in India have a poorer nutrition profile and chemotherapy tolerance than western patients. Therefore, we present this study that investigated the efficacy of DOF regimen in Indian population which gave a median PFS of 7 months and a median OS of 16 months which correlates well with the available data on DOF from the international trials as discussed above. The efficacy of DOF regimen in Indian patients is comparable to that seen in the international studies. To conclude, DOF regimen can be effectively used in Indian patients for the treatment of metastatic gastric adenocarcinoma with good results, and may be considered a first-line regimen for this indication.
ECOG, Eastern Cooperative Oncology Group; PS, performance status
CR, complete response; DOF, docetaxel, oxaliplatin, and fluorouracil; PD, progressive disease; PR, partial response; SD, stable disease
Citation: Patil. P (2023), Improved survival with DOF regimen for the treatment of metastatic gastric adenocarcinoma �??�?�¢�?�¢?�?�¬�?�¢?? A single-center experience J Can Sci Res 8:532
Received: 27-Dec-2022, Manuscript No. JCSR-22-6638; Editor assigned: 29-Dec-2023, Pre QC No. JCSR-22-6638 (PQ); Reviewed: 12-Jan-2023, QC No. JCSR-22-6638; Revised: 19-Jan-2023, Manuscript No. JCSR-22-6638 (R); Published: 27-Jan-2023 , DOI: 10.35248/2576-1447.23.8.532
Copyright: © 2023 Patil. P. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original work is properly cited credited.