Journal of Plant Biochemistry & Physiology
Open Access
ISSN: 2329-9029
ISSN: 2329-9029
Research Article - (2019)Volume 7, Issue 4
Recently, tissue culture based in vitro selection have been studied as cost-effective feasible tool for developing stress-tolerant plants. Somaclonal variation created in an experiment conducted with two potatoes (Solanum tuberosum L.) cultivars Santana and Spunta to create somaclonal variation by repetitious subcultures on MS medium for the purpose of evaluating this somaclonal variation for biotic stress resistance, the two cultivars were inoculated in vitro with four concentrates 1 × 102, 1 × 104, 1 × 106 and 1 × 108 cfu/ml Pectobacterium atrosepticum. Both cultivars showed significant reduction of disease severity index (DSI) at highest inoculum level 1 × 108 cfu/ml between pre and post subculture. Santana cv. DSI was 51.17% and 61.5% improved significantly to 25.17% and 44.17% for calcium treated and non-treated respectively, while in Spunta cv. DSI was 54.00% and 62.33% improved significantly to 32.67% and 46.17% for calcium treated and non-treated respectively. The highest DSI recorded in pre-subculture non-treated with Ca were 33.23% and 35.16% for Santana and Spunta cvs. respectively while the lowest DSI recorded in post subculture treated with Ca were 11.97% and 15.20% for Santana and Spunta cvs. respectively. In vivo inoculation of in vitro survived plantlets with the same inoculum levels showed better performance of Santana cv. over Spunta cv. at 1 × 104 ,1 × 106 cfu/ml with (20.33%, 33.33% ) and (34.00%, 42.67% ) respectively. Calcium nitrate improved plants tolerance by reducing DSI significantly from 66.33% to 35.00% at 1 × 108 cfu/ml for non-treated and treated respectively.
In vitro screening; Pectobacterium ssp; Diseases resistance; Potato (Solanum tuberosum)
Potato (Solanum tuberosum L.) is one of the major tuber food crop plays a significant role in human nutrition worldwide and contributes to world food security to face hunger. It became the third most widely consumed plant by humans after wheat and rice and recently potato and its processed products have gradually become important trade goods as an important semi-manufactured product. It is an important cash crop widely cultivated throughout the world. Potato is a temperate or continental zone crop and is cultivated in many areas worldwide. Potato ranks the fourth most important food crop grown in the world in term production volume, exceeded by cereals crops maize (Zea mays L.), wheat (Triticum aestivum L.) and rice (Oryza sativa L.) respectively where about 388 million tons of potatoes are produced on nearly 45 million feddans (feddan=0.42 ha) of land [1,2].
In Egypt, potato crop has an important position among all vegetables, it ranks the fourth most important crop exceeded by wheat, maize and rice respectively, production trend and area is increasing annually since year 2000. About 22.3% of total area devoted for vegetable production is cultivated with potato. In addition, the total cultivation of potatoes reached 390,330 feddans which produce 4,325,478 tons of tubers with an average yield of 11.1 tons/feddan [2]. This crop is economically important to Egypt and any disturbance in its production affects severely its local and export trade.
In vitro selection based on tissue culture techniques showed feasible results as cost effective tool to develop stress-tolerant plants, through induction of genetic variation among cells, tissues and organs in cultured and regenerated plants which is known as somaclonal variation [3].
Recently, a bacterial disease emerged as important biotic stress namely Pectobacterium atrosepticum (Pa) formerly known as Erwinia carotovora subsp. Atroseptica, the causal organism of blackleg disease. Symptoms on plant level is characterized by slimy black rotten lesions up the stems in wet conditions or stunting, yellowing and wilting of stems and leaves if conditions are dry. On cellular level it produces pectolytic plant cell wall degrading enzymes (polygalacturonase and pectin lyase) which allow infiltration and produce macerated tissues that induce electrolyte leakage and cell death and releasing bacterial inoculum which spreads to other tubers either in in the field, transit or storage periods [4]. Blackleg impose a major threat to agriculture by affecting drastically the production capacity of the crop causing quantitative and qualitative losses reaching 60% which represent particular importance to the processing sector where tubers are commonly stored for extended periods prior to factory processing [5]. Therefore, a lot of efforts were paid to develop stress resistant plants which is important to increase crop productivity.
In vitro plantlets are exposed to many extreme conditions during their culture imposing stress due explanting during cutting from the mother plant, hormonal effect and the nature of the culture medium, plantlets then respond by activating a number of variable systemic defense mechanisms and developmental responses [6]. This variation (somaclonal variation) either resulted from preexisting genetic variation within the explant or variation induced during the tissue culture phase can be exploited to generate disease-resistant clones when no known source of natural resistance exists either in cultivated or wild relatives [7]. In vitro selection is based on the induction of genetic variation among cells, tissues and/or organs in cultured and regenerated plants, based on this, plants resistant to the biotic stresses can be acquired by applying the selecting agents such as pathogen culture filtrate, phytotoxin or pathogen itself (for disease resistance) in different ways, only the explants capable of sustaining such environments and survive are selected [8].
It has been found that calcium supply is essential in mineral nutrition associated with potato plant defense, it is increasing the resistance of potato stems and tubers to pectolytic enzymes maceration by calcium precipitation as calcium pectate and thus making the cell wall rigid and enhances the structural integrity of cell walls and membranes of the plants, hence reduces disease severity of blackleg and soft rot in potato where calcium nitrate was effective in reducing blackleg disease by more than 20% [9]. The objective of this study is aimed to screening for new variants in two potato cultivars (Santana and Spunta) through somaclonal variation resistant to biotic stresses represented by bacteria Pectobacterium atrosepticum in this study, the causal organism of blackleg disease in Potatoes.
Plant material
Two potato cultivars Santana and Spunta were used as plant materials, the first cultivar is imported from Stet Holland Co., Netherland and Spunta obtained from the local market. Samples were collected from local seed stocks. Medium sizes 35-45 mm, pre sprouted tubers were selected randomly.
Tuber sprouts initiation
The collected two cultivars (Santana and Spunta) tubers were brushed dry to remove adhered soil, mechanical impurities and soilborne diseases. They were cleaned with water and soap for 10 min, washed thoroughly for 5 times with running tap water for 15 min. Seed potatoes were maintained at room temperature under humid conditions and total darkness for sprouting [10]. Rapid development of firm strong sprouts was encouraged by transferring tubers from dark to indirect (diffused) light.
Sterilization of initial explants
The sprouts were collected at 0.5-1.0 cm length and the tubers were kept in place as usual to get further sprouting. Excised sprouts were removed from tubers using a sterile scalpel, washed thoroughly under flow of running tap water for 20 minutes prior to sterilization in the laminar airflow cabinet and submersed in 20% commercial Chlorox containing approximately 1% sodium hypochlorite with a drop of Tween 20 for 15 min. After 3-4 times rinsing in sterile distilled water, the shoot buds were treated with 70% ethyl alcohol for one minute and washed three times in sterile distilled water to be ready for culturing [11].
Shoot initiation and multiplication medium
The sterilized sprouts were transferred to MS medium as shoot initiation medium and supplemented with a common level (2 mg/l) of N6-benzyladenine (BA) [12,13]. Sterilized sprouts were implanted in 150 × 25 mm glass culture tubes, one sprout/tube on the initiation medium, after 4 weeks the sprouts developed into plantlets of the 2 cultivars were produced in vitro through standard stem nodal multiplication method and subcultured every 4 weeks on fresh multiplication medium in 250 ml glass jars; each with 40 ml. This process was continued for 5 subcultures until complete plantlets were obtained in sufficient numbers (plantlets stock). All steps were under the same cultural conditions and incubated in a growth chamber at 20 ± 2°C under a photoperiod of 16/8 h (light/dark cycles) with a light intensity of 2500 to 3000 lux using cool white fluorescent lamps [14].
Media preparation
Tissue culture medium (MS 1962): (MS) medium for shoot multiplication used with 30 g/l sucrose and solidified with 0.6% agar as culture initiation media supplemented with 2 mg/l BA. The pH of the media was adjusted to 5.8 prior to the addition of Agar. Dispensed media were autoclaved at 121°C for 20 min.
Nutrient agar (NA) and Crystal violet pectate (CVP) medium: Nutrient agar and Crystal violet pectate medium is available as a premixed powder from Oxoid and HiMedia respectively, suspend in distilled water and bring volume to 1.0 L. Mix thoroughly on hot plate stirrer and dissolving completely adjust the pH to 6.9 ± 0.2 and then sterilize it by autoclave on 121°C for 20 min. Pour the autoclaved media into petri dishes 10 ml per each under aseptic conditions.
Preparation of Bacterial inoculum
Sampling and isolation of blackleg: The blackleg typical symptoms infected potato plants were collected from the field. These samples were transferred to the laboratory in polyethylene boxes for further studies. Tubers were washed, dried and peeled to include the heel (stolon) end, then using a sterile scalpel a small plug (about 1 cm deep and wide) of tissue was removed from the stolon end of each tuber in the sample [15]. Then surface sterilized 70% ethanol for 2 minutes followed by two successive rinses in sterile distilled water then homogenized in saline solution (0.85% NaCl) and streaked onto nutrient agar media (NA) and incubated at 28°C for 48 hr. Predominant colonies were selected purified re-streaking on to the same media, then streaked on crystal violate pectate (CVP) medium as selective medium and incubated at 28°C for 48 hours [16].
Identification of bacterial isolates: Bacterial isolates were identified by Department of Plant Pathology, Faculty of Agriculture, Ain Shams University as Pectobacterium atrosepticum using the Biolog Identification System GN MicroPlate system (developed by Biolog, Inc., Hayward, USA). The test yields a characteristic metabolic fingerprint. The microplates were incubated for 4 and 24 hours. Biolog’s MicroLog 2 4.2 software was used to identify the bacterium from its metabolic pattern. The calculations for the identification of bacteria as to genus, species and other taxonomic units are based on similarity indices [17].
Plantlets inoculation
In vitro Inoculation: Nodes of the two cultivars (Santana and Spunta) each were splited into two groups, the first group were multiplied on MS medium supplemented with 2 mg/l Calcium pantothenate while the second group were multiplied on Calcium pantothenate free MS medium. One month old plantlets were inoculated by sterile inoculation needle dipped into bacterial suspension of the four concentrates 1 × 102,1 × 104,1 × 106 and 1 × 108 colony forming units (cfu/ml) Pectobacterium atrosepticum adjusted by haemocytometer counting chamber and pressed into the crown of each plantlet, each treatment had 3 replicates each replicate includes 10 plantlets. Parameter of disease severity index was measured after 6, 9, 12 and 15 days post inoculation (dpi) [18].
In vivo inoculation: The best survived in vitro post subculture calcium treated plantlets at 1 × 108 cfu/ml of the two cultivars showed moderately resistant trait were acclimatized in trays on peat moss substrate for 20 days in greenhouse, then plants were inoculated by piercing the stem with a sterile tooth pick contaminated with Pectobacterium atrosepticum suspension with same concentrations with same replicates mentioned formerly 5 cm above the soil level. Inoculation sites were immediately wrapped with parafilm to prevent desiccation. Plants were incubated at 25°C at high humidity and blackleg lesions were evaluated after 21 dpi [19].
Assessment of disease resistance of the cultivars against Pectobacterium atrosepticum
Under in vitro condition: The symptoms of individual response of in vitro plantlets post bacterial infection were recorded and evaluated as the percentage of wilted leaves of the plantlets at time intervals of 6, 9, 12 and 15 dpi. (DSI) obtained from in vitro evaluation assays and wilting symptoms were scored based on 0 to 5 scale [18,19]. As following: 0=no symptoms, 1=0- 25% leaf wilting, 2=26%-50% leaf wilting, 3=51%–75% leaf wilting,4=76-100% leaf wilting and 5=dead plants as shown in (Figure 1).
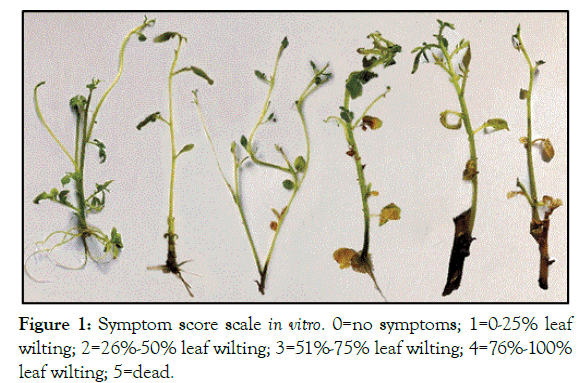
Figure 1: Symptom score scale in vitro. 0=no symptoms; 1=0-25% leaf wilting; 2=26%-50% leaf wilting; 3=51%-75% leaf wilting; 4=76%-100% leaf wilting; 5=dead.
(DSI) for Pectobacterium atrosepticum disease was calculated according to the formula below [20].
DSI=(0 × a)+(1× b)+(2 × c)+(3 × d)+(4 × e)+(5 × f) × 100/Y × 5
where a, b, c, d, e and f are the number of plantlets examined which fall into the categories
0, 1, 2, 3, 4 and 5, respectively [21].
Where:
Y=Total number of plantlets
5=Scale range
a=number of plantlets in grade 0
b=number of plantlets in grade 1
c=number of plantlets in grade 2
d=number of plantlets in grade 3
e=number of plantlets in grade 4
f=number of plantlets in grade 5
Plants were categorized as:
Resistant (R) when DSI between 0-20%, Moderately resistant (MR) when DSI between 20-40%, Moderately susceptible (MS) when DSI between 40 and 60%, Susceptible (S) when DSI more than 60% [22].
Under in vivo condition
Initial symptoms of black stripped color on stem base were recorded and evaluated for the 10 acclimatized plantlets per each cultivar as percentage of degree of wilting stems. A rating scale of 0 to 5 was used to indicate the degree of stem wilting as following:
0=No disease symptoms.
1=2-5 cm of the stem was striped.
2=5-8 cm of the stem was striped.
3=8-11 cm of the stem was striped.
4 ≥ 11 cm of the stem was striped.
5=Whole plant wilting.
Statistical Data Analysis
Disease severity index was calculated according aforementioned formula, Statistical analysis performed. Data were analyzed using the SPSS® program. Analysis of variance was determined using the general linear model procedures and means were separated with LSD test.
In vitro screening for resistance
Billions of dollars are spent each year on management of plant diseases and yet it is estimated that plant diseases cause yield loss in food and cash crop, these losses may be limited by the use of fungicides, sanitation practices, crop rotation or by the use of disease-tolerant cultivates [23]. Pectobacterium pathogens has been studied for over a century to promote rot, soft rot pathogens employ a wide range of plant cell wall degrading enzymes to disrupt and metabolize plant cells. In this study the two cultivars Santana and Spunta showed significant difference regarding DSI resulted from blackleg disease symptoms assessment when plantlets of both cultivars were compared pre and post being submitted to in vitro sub-culturing.
In the in vitro screening of both cultivars for Pectobacterium atrosepticum resistance under different selection levels of bacterial inoculum and calcium; both inoculated potato cultivars developed blackleg symptoms with various degrees when pre and post sub-culturing were compared. Pre sub-culture revealed higher DSI values which increased ascendingly in corresponding to inoculum level, this was in accordance with Toth while in post sub-culture was less [24].
In both cultivars DSI revealed significant differences across interacted factors (Interaction among culture type, inoculum levels and calcium treatment) where pre and post subculture revealed the highest and lowest DSI respectively in both cultivars. Each inoculation level of pre subculture either treated or nontreated with calcium varied significantly form the corresponding inoculation level of the post subculture (Tables 1 and 2).
| Culture type | Bacterial concentration in CFU/ml | Ca | 0 | Infected | Total | DSI% | Resistance level |
|---|---|---|---|---|---|---|---|
| Pre | Control | + | 8.25 | 1.75 | 10 | 4.83 | R |
| - | 6.83 | 3.17 | 10 | 13.33 | R | ||
| 1 × 102 | + | 7.00 | 3.00 | 10 | 9.83 | R | |
| - | 6.17 | 3.83 | 10 | 17.17 | R | ||
| 1 × 104 | + | 6.00 | 4.00 | 10 | 14.50 | R | |
| - | 5.25 | 4.75 | 10 | 25.50 | MR | ||
| 1 × 106 | + | 3.92 | 6.08 | 10 | 25.33 | MR | |
| - | 2.00 | 8.00 | 10 | 48.67 | MS | ||
| 1 × 108 | + | 1.00 | 9.00 | 10 | 51.17 | MS | |
| - | 0.42 | 9.58 | 10 | 61.50 | S | ||
| Post | Control | + | 9.42 | 0.58 | 10 | 1.33 | R |
| - | 8.67 | 1.33 | 10 | 4.00 | R | ||
| 1 × 102 | + | 8.83 | 1.75 | 10 | 3.00 | R | |
| - | 7.33 | 2.42 | 10 | 7.50 | R | ||
| 1 × 104 | + | 7.00 | 3.00 | 10 | 9.33 | R | |
| - | 5.83 | 4.17 | 10 | 16.33 | R | ||
| 1 × 106 | + | 3.58 | 6.42 | 10 | 21.00 | MR | |
| - | 1.58 | 8.42 | 10 | 37.67 | MR | ||
| 1 × 108 | + | 2.33 | 7.67 | 10 | 25.17 | MR | |
| - | 0.92 | 9.08 | 10 | 44.17 | MS | ||
| LSD | 3.149 | ||||||
Ca: (+) treated, (-) non-treated, (R) resistant, (MR) moderately resistant, (MS) moderately susceptible and (S) susceptible.
Table 1: Interaction among culture type, inoculum levels and calcium levels on DSI of Santana cv.
| Culture type | Bacterial concentration in CFU/ml | Ca | 0 | Infected | Total | DSI% | Resistance level |
|---|---|---|---|---|---|---|---|
| Pre | Control | + | 7.83 | 2.17 | 10 | 6.83 | R |
| - | 6.75 | 3.33 | 10 | 14.47 | R | ||
| 1 × 102 | + | 6.83 | 3.17 | 10 | 12.50 | R | |
| - | 6.08 | 3.92 | 10 | 20.50 | MR | ||
| 1 × 104 | + | 6.08 | 3.92 | 10 | 14.50 | R | |
| - | 5.17 | 4.83 | 10 | 29.33 | MR | ||
| 1 × 106 | + | 4.08 | 5.92 | 10 | 27.00 | MR | |
| - | 2.00 | 8.00 | 10 | 49.17 | MS | ||
| 1 × 108 | + | 1.17 | 8.83 | 10 | 54.00 | MS | |
| - | 0.50 | 9.50 | 10 | 62.33 | S | ||
| Post | Control | + | 9.33 | 0.67 | 10 | 1.50 | R |
| - | 8.75 | 1.25 | 10 | 3.83 | R | ||
| 1 × 102 | + | 8.83 | 1.67 | 10 | 3.33 | R | |
| - | 7.33 | 2.42 | 10 | 7.83 | R | ||
| 1 × 104 | + | 6.67 | 3.33 | 10 | 13.67 | R | |
| - | 5.83 | 4.17 | 10 | 19.83 | R | ||
| 1 × 106 | + | 3.42 | 6.58 | 10 | 24.83 | MR | |
| - | 1.58 | 8.42 | 10 | 42.00 | MS | ||
| 1 × 108 | + | 1.50 | 8.50 | 10 | 32.67 | MR | |
| - | 0.67 | 9.33 | 10 | 46.17 | MS | ||
| LSD | 3.242 | ||||||
Ca: (+) treated, (-) non-treated, (R) resistant, (MR) moderately resistant, (MS) moderately susceptible and (S) susceptible.
Table 2: Interaction among culture type, inoculum levels and calcium levels on DSI of Spunta cv.
Two exceptions where non-significant difference can be found, the first one in Spunta cv. between pre and post subculture when treated with calcium at 1 × 104 cfu/ml and similarly at 1 × 106 cfu/ml, while the second one where pre subculture either treated or non-treated with calcium at 1 × 102 cfu/ml showed no significant difference form post subculture treated and nontreated at and 1 × 104 cfu/ml in both cultivars, which reflect the improved tolerance level, where almost same DSI could be tolerated at higher inoculum level. The reason behind nonsignificant difference shown at post subculture in both cultivars between plantlets of control (non-treated with calcium) and inoculum level of 1 × 102 cfu/ml (treated with calcium) could be understood in the light of Toth [24] and Yao and Allen [25] who considered that 1 × 102-1 × 103 cfu /ml as low inoculum level.
Calcium has played a major role in suppressing the disease progress in post subculture which is found in the significant difference between pre and post subculture either treated or nontreated with calcium for both cultivars (Tables 3 and 4).
| Culture type | Calcium | 0 | Infected | Total | DSI% | Resistance level |
|---|---|---|---|---|---|---|
| Pre | Treated | 5.20 | 4.80 | 10 | 22.97 | MR |
| Non-Treated | 4.10 | 5.92 | 10 | 35.16 | MR | |
| Post | Treated | 5.95 | 4.15 | 10 | 15.20 | R |
| Non-Treated | 4.83 | 5.12 | 10 | 23.93 | MR | |
| LSD | 1.450 |
(R) resistant, (MR) moderately resistant, (MS) moderately susceptible and (S) susceptible.
Table 3: Effect of in vitro Interaction between culture type and calcium levels on DSI of Santana cv.
| Culture type | Calcium | 0 | Infected | Total | DSI% | Resistance level |
|---|---|---|---|---|---|---|
| Pre | Treated | 5.23 | 4.77 | 10 | 21.13 | MR |
| Non-treated | 4.13 | 5.87 | 10 | 33.23 | MR | |
| Post | Treated | 6.23 | 3.88 | 10 | 11.97 | R |
| Non-treated | 4.87 | 5.08 | 10 | 21.93 | MR | |
| LSD | 1.408 |
(R) resistant, (MR) moderately resistant, (MS) moderately susceptible and (S) susceptible.
DSI ranged from 11.97% up to 33.23% and from 15.20% up to 35.16% for Santana cv. and Spunta cv. respectively, Where the lowest DSI was confined to post subculture plantlets treated with calcium and the highest was confined to pre subculture plantlets non-treated with calcium for both cultivars, this comes in accordance with McGuire and Kelman [26] and McMillan et al. [27] findings, that resistance to soft rots diseases has been associated with high calcium levels and pectin methylation of plant cell walls (Figures 2 and 3).

Figure 2: Disease severity on Santana cv. treated with Ca (A) and nontreated with Ca (B).

Figure 3: Disease severity on Spunta cv. treated with Ca (A) and nontreated with Ca (B).
In the same context, pre and post subculture interaction with different inoculum levels revealed significant difference for both cultivars. In Santana cv. pre and post subculture plantlets resulted in 13.5% and 12.83% when inoculated with 1 × 102 and 1 × 104 cfu/ml respectively (Figure 4).
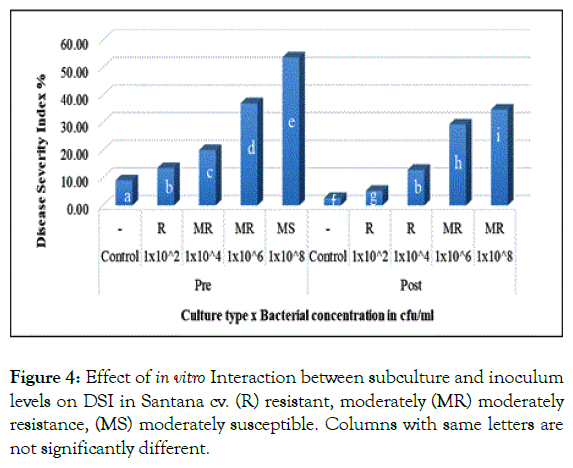
Figure 4: Effect of in vitro Interaction between subculture and inoculum levels on DSI in Santana cv. (R) resistant, moderately (MR) moderately resistance, (MS) moderately susceptible. Columns with same letters are not significantly different.
Showing no significant difference, similarly in Spunta cv. pre and post subculture plantlets which first inoculated with 1 × 102 and 1 × 104 cfu/ml resulted in 16.5% and 16.75% respectively and secondly with 1 × 106 and 1 × 108 cfu/ml resulting in 38.08% and 39.42% respectively (Figure 5). This shift from pre subculture with low inoculum level to post subculture with higher inoculum level is considered an improvement of resistance due to sub-culturing and could be explained in the light of Ghorbani et al. [28] who mentioned that source of resistance against blackleg disease was found available in some of potato cultivars, accordingly, based on the variation in resistance scores observed between both pre and post sub-culturing could be attributed to somaclonal variation created during repetitious subcultures as described by Shepherd and Ghartavol et al. [29,30].
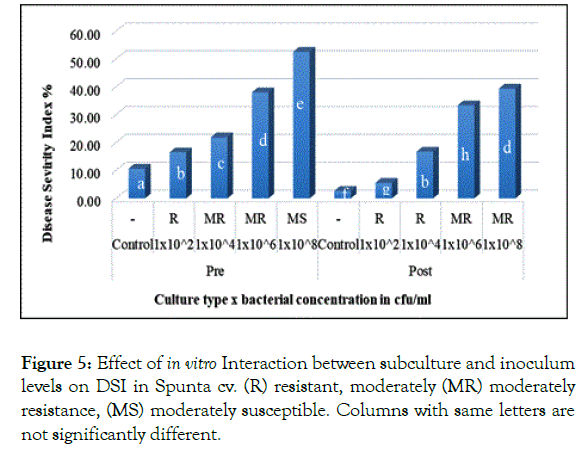
Figure 5: Effect of in vitro Interaction between subculture and inoculum levels on DSI in Spunta cv. (R) resistant, moderately (MR) moderately resistance, (MS) moderately susceptible. Columns with same letters are not significantly different.
Interaction of calcium treatment with bacterial inoculum levels revealed significant effect of calcium in reducing DSI. In Santana cv. plantlets either non-treated or treated with calcium when inoculated with 1 × 102 and 1 × 104 cfu/ml resulted in 12.33% and 11.92% respectively showing no significant difference (Figure 6), in parallel, Spunta cv. calcium non-treated plantlets inoculated with 1 × 102 and 1 × 104 cfu/ml resulted in 14.17% and 24.58% respectively didn’t differ significantly from calcium treated plantlets when inoculated with 1 × 104 and 1 × 106 which resulted in 14.08% and 25.92% respectively (Figure 7) which reflect the improvement of resistance at high inoculum level with calcium treatment when compared with lower inoculum level without calcium treatment at the same DSI.
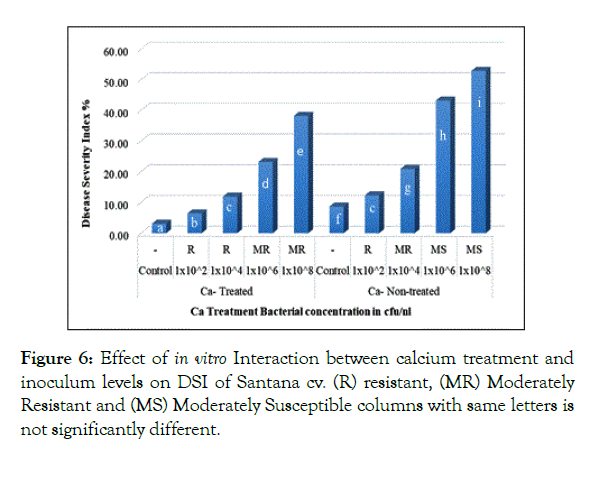
Figure 6: Effect of in vitro Interaction between calcium treatment and inoculum levels on DSI of Santana cv. (R) resistant, (MR) Moderately Resistant and (MS) Moderately Susceptible columns with same letters is not significantly different.
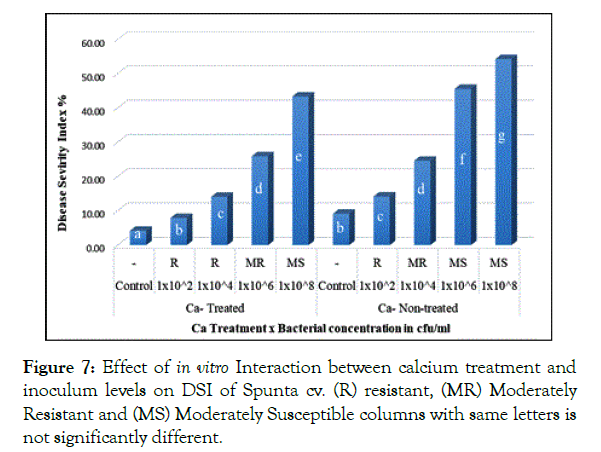
Figure 7: Effect of in vitro Interaction between calcium treatment and inoculum levels on DSI of Spunta cv. (R) resistant, (MR) Moderately Resistant and (MS) Moderately Susceptible columns with same letters is not significantly different.
In vivo resistance screening
In vivo inoculated cultivars developed blackleg symptoms with various degrees. The result of the evaluation revealed the ability of calcium in treated plants to reduce the DSI which showed significantly lower values than non-treated plants. Across the two varieties, calcium significantly improved resistance of treated plants at inoculum level of 1 × 104, 1 × 106 and 1 × 108 cfu/ml in compare ton on-treated plants at the same inoculation levels respectively (Figures 8 and 9).
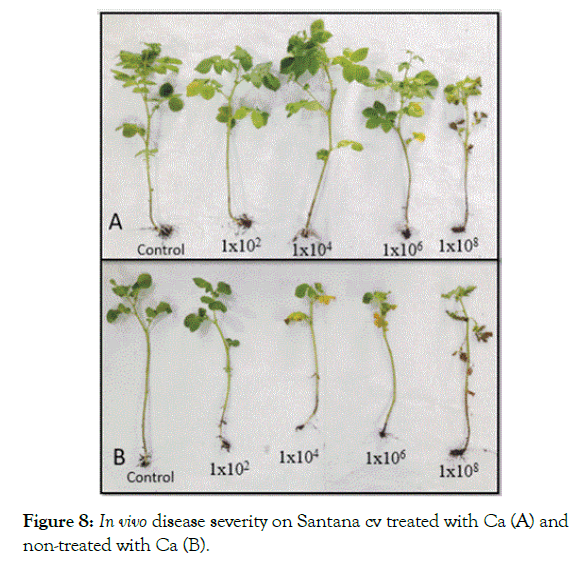
Figure 8: In vivo disease severity on Santana cv treated with Ca (A) and non-treated with Ca (B).
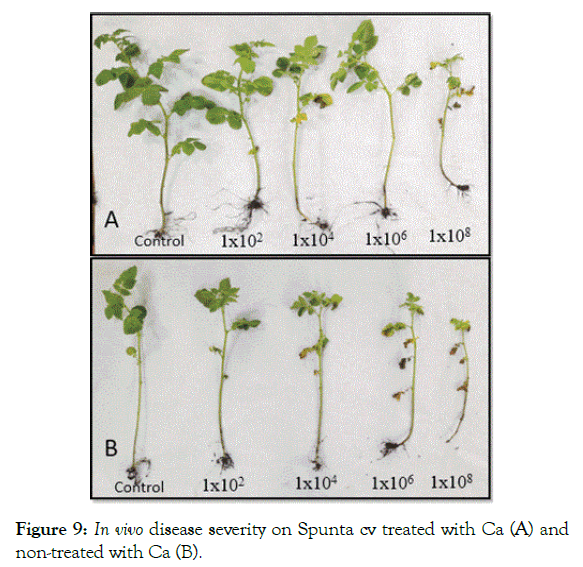
Figure 9: In vivo disease severity on Spunta cv treated with Ca (A) and non-treated with Ca (B).
This reflect the ability of calcium treated plants to resist higher infection levels while keeping the DSI to a similar level of those calcium non-treated plants which couldn’t resist at lower infection levels (Table 5).
| Calcium | Bacterial concentration CFU/ml | 0 | Infected | Total | DSI% | Resistance level |
|---|---|---|---|---|---|---|
| Treated | Control | 8.67 | 1.33 | 10 | 3.00 | - |
| 1 × 102 | 6.67 | 3.33 | 10 | 9.33 | R | |
| 1 × 104 | 5.33 | 4.67 | 10 | 16.33 | R | |
| 1 × 106 | 3.50 | 6.50 | 10 | 27.00 | MR | |
| 1 × 108 | 2.00 | 8.00 | 10 | 35.00 | MR | |
| Non-treated | Control | 6.67 | 3.33 | 10 | 13.33 | - |
| 1 × 102 | 5.50 | 4.50 | 10 | 16.00 | R | |
| 1 × 104 | 3.67 | 6.33 | 10 | 38.00 | MR | |
| 1 × 106 | 2.50 | 7.50 | 10 | 49.00 | MS | |
| 1 × 108 | 1.00 | 9.00 | 10 | 66.33 | S | |
| LSD | 6.817 | |||||
(R) resistant, (MR) moderately resistant, (MS) moderately susceptible and (S) susceptible.
Table 5: Effect of in vivo Interaction of inoculum levels with Calcium levels on DSI.
On the other hand, treated plants when inoculated with 1 × 102 and 1 × 104 cfu/ml showed no significant difference form nontreated control which is in accordance with Toth et al. [24] and Yao and Allen [25] when reported that 102-103 cfu/ml considered to be low inoculum level and doesn’t give rise to the level of pathogenicity, plus blackleg disease doesn’t develop rapidly below the threshold level for disease development which is 103 cfu/ml as described by Perombelon [31].
Similarly, calcium succeeded to reduce the DSI across all inoculum levels for both varieties, interaction between calcium levels and cultivars revealed DSI ranging from 16.00% up to 41.87% for treated Santana cv. and non-treated Spunta cv. respectively (Table 6).
| Calcium | Variety | 0 | Infected | Total | DSI% | Resistance level |
|---|---|---|---|---|---|---|
| Treated | Santana | 5.87 | 4.13 | 10 | 16.00 | R |
| Spunta | 4.60 | 5.40 | 10 | 20.27 | MR | |
| Non-treated | Santana | 4.67 | 5.33 | 10 | 31.20 | MR |
| Spunta | 3.07 | 6.93 | 10 | 41.87 | MS | |
| LSD | 4.312 | |||||
R) resistant, (MR) moderately resistant and (MS) moderately susceptible.
Table 6: Effect of in vivo Interaction of cultivars with Calcium levels on DSI.
In vivo DSI was affected by calcium nitrate as soil amendments which was effective in reducing blackleg disease as reported by Ngadze [9]. This is in complying with Flego et al. [32]. When reported that calcium enhancing resistance agianst plant tissue macerating bacteria using pectolytic enzymes.
The effect of interaction between cultivars and inoculum level showed insignificant difference when inoculated with (1 × 102 cfu/ml) which resulted in lowest DSI (10.67% and 14.67%) for Santana and Spunta cvs. respectively, while the highest DSI showed by Spunta plants inoculated with 1 × 108 cfu/ml resulted in 53.67%. Spunta plants inoculated with 1x106 cfu/ml resulted in (42.67%) showed insignificant difference form Santana plants inoculated with 1x108 cfu/ml resulted in (47.67%) respectively (Figure 10) demonstrating the tolerance of Santana cv. at higher inoculum levels in compare to Spunta cv. at the same DSI. The superiority of Santana cultivar over Spunta comply with Khlaif and Wreikat [33] who reported the high susceptibility found in Spunta cultivar due to its low calcium content.
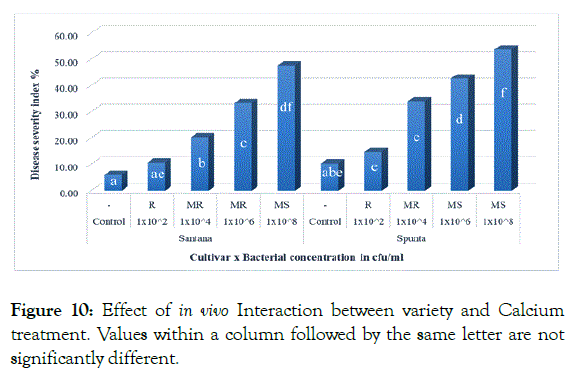
Figure 10: Effect of in vivo Interaction between variety and Calcium treatment. Values within a column followed by the same letter are not significantly different.
Breeding potato cultivars resistant to Pectobacterium spp. would reduce losses caused by these pathogens. Tubers of potato cultivars vary in their relative susceptibility to Pectobacterium spp. but none of the common cultivars are considered to be highly resistant. Many wild species of Solanum have resistance to Pectobacterium spp. Chemical control is known to be not effective in controlling them that’s why control strategies rely on the use of resistant cultivars, good agronomic practices such as planting certified disease-free seed, planting in well-drained soil and good sanitation. Pectobacterium atrosepticum almost exclusively infects potato, causing blackleg of the stem and tuber soft rot. Regarding the different purposes of the two cultivars and from the foregoing discussion it may be concluded that the Santana and Spunta cultivars post subculture were noted to have lower DSI when compared with post subculture, this reflects the improvement occurred through somaclonal variation.
It seems this variation may have improved calcium uptake to build calcium pectate responsible for plant cells walls integrity which is clearly shown in the significant difference between treated and non-treated plants. Calcium accumulation in potato tubers and uptake thresholds differs from cultivar to another based on their genetic capacity, accordingly improvement of tuber calcium can be done through plant breeding. Considering that Spunta cv. is one of Santana cv. parents (Spunta x VK69-491) may explain to some extent the partial similarity of performance in results. Generally, the in vivo trial resulted in better performance of Santana cv. where it resisted inoculum level of 1 x 106 cfu/ml by showing moderately resistance reaction while Spunta showed moderately susceptible one. Above findings indicated that resistance trait could be well improved by creating somaclonal variation followed by imposing selection factor and select the survivor variants.
Citation: Aboshama HM, Atwa MM (2019) In vitro Evaluation of Somaclonal Variation of Two Potato Cultivars Santana and Spunta for Resistance against Bacterial Blackleg Pectobacterium atrosepticum. J Plant Biochem Physiol 7: 243. doi: 10.35248/2329-9029.19.7.243
Received: 11-Nov-2019 Accepted: 25-Nov-2019 Published: 02-Dec-2019
Copyright: © 2019 Aboshama HM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.