Fungal Genomics & Biology
Open Access
ISSN: 2165-8056
ISSN: 2165-8056
Research Article - (2022)Volume 12, Issue 6
Candidiasis is a fungal infection (mycosis) caused by an opportunistic yeast with only 8-10 pathogenic species. In spite of the multiple classes of antifungal drugs available to combat these infections, the treatment is hampered by drug toxicity, tolerability, emergence of drug resistant isolates and many more. Combination therapy has been suggested as a possible approach to improve these treatment outcomes. This study is, therefore, aimed at the analysis of the sensitivity of Candida isolates obtained from patients towards different classes of drugs affecting multiple pathways in the pathogen life cycle. Four different individual drugs with different mechanisms of action and six different combinations of these drugs at three different proportions (1:1, 1:3, and 3:1) were used for the study. All the drug sensitivity assays were performed by the agar well diffusion method. The results were statistically analyzed using Prism software. Results have shown synergistic effects of drug combinations on the sensitivity of the isolates without much variation in the stoichiometric ratios, thereby providing an alternative to monotherapy to combat the emergence of drug resistant isolates and drying existing drug pipelines.
Candidiasis; Isolates; Drug combinations; Statistical analysis
The last few years have witnessed progressive increase in mycotic infections which are one of the primary causes of morbidity and mortality especially in immunocompromised and severely ill patients. Candida is a fungal pathogen which is usually a member of normal flora but transits to pathogenic form by several virulence factors like adherence to host tissues and medical devices, secretion of extracellular hydrolytic enzymes etc. [1]. The species is responsible for various clinical manifestations including overgrowth as well as life threatening disseminated infections. Candida species have been reported as the fourth most common nosocomial bloodstream pathogen by National Nosocomial Infections Surveillance System (NNISS) with a mortality rate of about 45% [1]. Till date, more than 150 species of Candidaare known with only 10 species responsible for the disease with C. albicans being responsible for majority of the infections. However, recent studies have shown the increase in incidences of infection due to non-albicans Candida species like Candida auris, Candida tropicalis, Candida glabrata, Candida parapsilosis, Candida krusei, and Candida lusitaniae [2,3]. The fatality of fungal infections in immune-compromised conditions has been recently observed with mucormycosis (black fungus) and Candida (white fungus) infections [4]. These infections spread worldwide in both COVID-19 positive patients as well as patients recovered from COVID-19. Studies have shown the presence of oropharyngeal Candidiasis in patients suffering from COVID-19 and C. auris infections in critically ill COVID-19 patients [5,6].
Several non-albicans species are inherently resistant or acquire resistance to commonly used antifungal drugs. Factors like severe immunosuppression, prematurity, use of broad-spectrum antibiotics, and usage of antimycotic drugs are associated with this change. Six different classes of antifungal drugs with different modes of action are available which selectively eliminate fungal pathogens from a host with minimal toxicity to the host. These include Azoles, Allylamines, Polyenes, Echinocandins, Nucleotide Analogue and Penicillin derivatives. Amphotericin and Azoles have been the choice of drugs for patients suffering from mucormycosis and Candida infections during the COVID-19 outbreak [7]. However, numerous recent studies have shown the emergence of drug resistant Candidastrains and isolates. This along with increase in disease causing species has led to a dire need for development of newer drug strategies. One of these novel drug strategies is the combinatorial therapy consisting of multiple drugs affecting different pathways which have proven to be much more efficacious than a single counterpart. It offers numerous advantages such as no or vey less toxicity analysis, lower treatment failure rate, lower case-fatality ratios, fewer side-effects than monotherapy, slower development of resistance, and thus less money needed for the development of new drugs[8]. Thus, in view of this, the present study is focused on determining the drug sensitivity profiles of Candida isolates obtained from patients in Rajasthan, India. Further, we have also analyzed the efficacy of drug combinations of multiple drugs in three different stoichiometric ratios towards these isolates. All the results were appropriately statistically analyzed. Our results have clearly shown that all drug combinations are much more efficacious than a single drug towards these isolates without much variation observed at different stoichiometric ratios.
Candida isolates and culture conditions
Candida isolates used in the study were kindly provided by MCRD-CIRD (Microbial Culture Repository Division–Centre for Innovation, Research and Department), Dr. B. Lal Clinical Laboratory Pvt. Ltd., Jaipur. These isolates were extracted from various infectious human samples such as urine, blood, pus and vaginal swabs obtained from Candidiasis patients in Rajasthan. Patient’s demographic details such as sex, age and clinical information were recorded and used for analysis. The study consists of a total of 17 isolates which were cultivated and maintained on YEPD medium (Hi Media Laboratories, Mumbai, India) (unless specified) at 30°C. The isolates were confirmed macroscopically by visualizing the colony morphology (creamy colony in color) on YEPD agar plates as well as microscopically using Indian ink (Nigrosin) staining.
Preparation of antifungal drugs and drug combinations
A total of four drugs, Itraconazole, Amphotericin B, Micafungin and Terbinafine, belonging to different classes were used in the study. All the drugs were prepared in DMSO as per CLSI guidelines. Itraconazole and Amphotericin B were used at a concentration of 13.5 mg/ml and 0.33 mg/ml while Micafungin and Terbinafine were used at a concentrati on of 50 mg/ml and 0.435 mg/ml. DMSO was used as a negative control for all the assays.
Depending on the results obtained from drug sensitivity assays using individual drugs, a total of six drug combinations were prepared at three different stoichiometric assays. The drug combinations used for the study are shown in Figure 1.
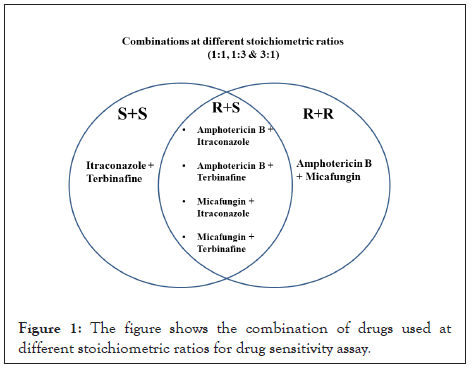
Figure 1: The figure shows the combination of drugs used at different stoichiometric ratios for drug sensitivity assay.
Antifungal drug sensitivity
All the drug sensitivity assays were performed as per standard procedures in accordance with NCCLS guidelines by agar well diffusion assay. Briefly, the isolates were primarily inoculated in Sabouraud Dextrose Broth (SDB) and incubated at 30°C for 24 hours. These grown cultures were then diluted to an O.D. of 0.2 and then swabbed on SDA plates. Wells were punctured in these plates and 50 µL of the prepared drug/drug combination was added into wells. Wells containing DMSO were used as negative control. Plates were incubated for 24 hours at 30°C and the clear zone obtained around the well, known as the zone of inhibition (ZOI), was measured by zone measuring scale.
Statistical analysis
The mean ZOI ± standard deviation of all isolates for a drug or a drug combination was calculated and was used for the comparison of the significance of drug combinations to individual drugs as well as combinations at various proportions. All the statistical analysis was done by unpaired t test using PRISM version 5.0.
Demographic analysis of the isolates
Demographic analysis of the isolates was done on the basis of gender, age group, sample type as well as species causing the infections are shown in Figure 2a. The details of the isolates obtained are summarized in the supporting data (Table S1). As it is evident from the results, Candida infections are not gender biased and affect both males and females are shown in Figure 2a. However, previous studies have shown that females are slightly more prone to Candida infections owing to their higher susceptibility towards UTIs (Urinary Tract Infections) [9,10]. The deviation in our results from the previous studies could be attributed to the smaller number of isolates used in the study [11,12]. The analysis of these infections in different age groups suggested almost similar infectivity rate in all age groups with slight increased rates in older age groups owing to weaker immune systems are shown in Figure 2b. This is well in agreement with previous studies suggesting that Candida infections mostly occur in immunocompromised patients, so it can occur in any age group who have weaker immune response [13-16]. Our results showed that majority of samples were obtained from the patient’s sputum and urine, indicating that Candida majorly affects respiratory and urinary systems in the human body are shown in Figure 2c. It is also correlated from previous studies that the organism majorly affects lungs causing diseases like pneumonitis [17,18]. Besides, Candida based UTI infections are also very prevalent mostly observed in females as vaginal candidiasis, vulvovaginal candidiasis caused by Candida albicans [19-21]. Isolates obtained from blood demonstrate the severity of the disease is shown in Figure 2c. Evidences have also suggested the occurrence of Candida pneumonia and Candiduria in severely immunocompromised individuals with disseminated disease[22,23]. Although the emergence of infections caused by non-albican species is increasing, the majority of the infections in this study were caused by albican species are shown in Figure 2d. This albican species bias has been observed previously as well from a number of studies [24-26].However, infections caused by non-albican species such as auris, glabrata, etc. are rising continuously which are also causing an increase in emergence of drug resistance[27-29].
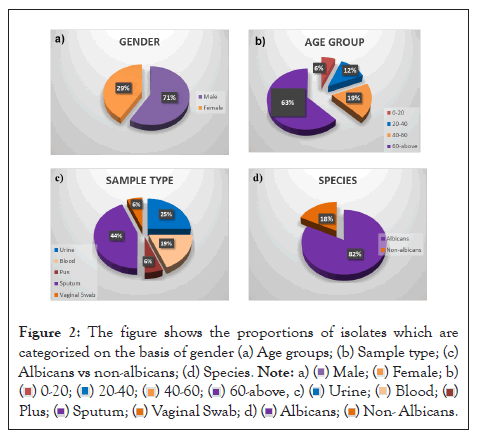
Figure 2: The figure shows the proportions of isolates which are categorized on the basis of gender (a) Age groups; (b) Sample type; (c) Albicans vs non-albicans; (d) Species.


Drug sensitivity of isolates towards monotherapeutic drugs
Four different antifungal drugs belonging to different classes were used for the study. Drug sensitivity analysis by standard well diffusion assay was done with DMSO as the negative control. Representative images of drug sensitivity assay are shown in the supporting data (Figure S1) and as per CLSI guidelines 17 mm ZOI was taken as the cut-off value. On the basis of results shown in Table 1, it is clear that all the isolates were sensitive to Amphotericin B and Micafungin while only 59% and 53% isolates were sensitive to Terbinafine and Itraconazole respectively. The sensitivity of the isolates towards Amphotericin B and Micafungin have also been previously reported to 99.5% and 100% respectively [30,31]. Similar results were observed with patients suffering from oral Candidiasis or autoimmune Candida dystrophy [32].Similarly, emerging resistance was observed for Candida isolates towards Itraconazole [31-33]. Also,emergence of resistant has been observed with isolates towards Terbinafine [34], in patients suffering from Balanoposthitis caused by Candida albicans [35]. These results along with previous studies clearly indicate the emergence of drug resistant isolates in the area under the study and elsewhere towards Azoles and Terbinafine. Thus, alternate options need to be evaluated to combat the infections caused by the pathogen.
| S.No. | Sample | Amphotericin | Terbinafine | ltraconazole | Micafungin |
|---|---|---|---|---|---|
| 1 | RPC 804 | 21 mm | 14 mm | 14 mm | 17 mm |
| 2 | C1654 | 20 mm | 12 mm | 10 mm | 19 mm |
| 3 | 37/1561 | 21 mm | 13 mm | 14 mm | 21 mm |
| 4 | RPC 937 | 20 mm | 16 mm | 16 mm | 23 mm |
| 5 | 64/316 | 22 mm | 0 mm | 10 mm | 19 mm |
| 6 | 20/519 | 19 mm | 14 mm | 17 mm | 21 mm |
| 7 | MNC 2175 | 26 mm | 26 mm | 20 mm | 21 mm |
| 8 | KNC 32 | 21 mm | 23 mm | 0 mm | 29 mm |
| 9 | C1805 | 20 mm | 16 mm | 10 mm | 17 mm |
| 10 | 330/1994 | 23 mm | 24 mm | 31 mm | 28 mm |
| 11 | C2325 | 26 mm | 22 mm | 18 mm | 27 mm |
| 12 | C2262 | 27 mm | 25 mm | 18 mm | 26 mm |
| 13 | C2264 | 26 mm | 25 mm | 19 mm | 29 mm |
| 14 | C2321 | 25 mm | 21 mm | 20 mm | 30 mm |
| 15 | C386 | 24 mm | 25 mm | 2 mm | 29 mm |
| 16 | C128 | 21 mm | 26 mm | 21 mm | 30 mm |
| 17 | C2554 | 25 mm | 30 mm | 18 mm | 31 mm |
| Percent Sensitivitv | 100 (17/17) | 59 (10/17) | 53 (10/17) | 100 (17/17) |
Note: Resistant isolates are highlighted in red.
Table 1: Protein content of different strains of Pleurotuscystidiosus mushrooms (g/100 g of dried sample).
Drug sensitivity of isolates towards combinatorial therapy
Studies so far have indicated that individual drugs may not be the best choice in the current scenario. Multiple studies have illustrated drug combinations to be more efficacious preventing the emergence of resistant strains, lowering the concentration of drugs and treating critically ill patients rapidly[36]. Not just towards Candida infections, combinatorial drugs have proven to be effective in other infections as well such as Chaetomium species, invasive aspergillosis, invasive mold diseases in hematologic patients [37-39]. We, therefore, have analyzed the drug sensitivity of isolates towards drug combinations at three different stoichiometric ratios. The drugs to which more than and less than 60% isolates were sensitive were considered as S and R respectively. Representative images of drug sensitivity assay are shown in the supporting data (Figure S2). The drug combinations were used to evaluate the synergistic effects of drugs in combinations. On the basis of results shown in Tables 2-4, Itraconazole and Terbinafine were individually not effective to all the isolates but their combination was especially effective when used at 1:1 combination. Similarly, all the other five combinations showed statistically significant synergistic effects towards the growth of the isolates. These results are well in agreement with numerous previous studies which have been discussed here.
| Zone of inhibition | |||||||
|---|---|---|---|---|---|---|---|
| S.No. | Sample | l+T | M+T | A+T | l+M | A+I | A+M |
| 1 | RPC 804 | 25 mm | 30 mm | 28 mm | 28 mm | 25 mm | 29 mm |
| 2 | C1654 | 23 mm | 29 mm | 18 mm | 27 mm | 20 mm | 28 mm |
| 3 | 37/1561 | 25 mm | 28 mm | 22 mm | 29 mm | 21 mm | 24 mm |
| 4 | RPC 937 | 27 mm | 31 mm | 21 mm | 27 mm | 24 mm | 25 mm |
| 5 | 64/316 | 26 mm | 28 mm | 19 mm | 26 mm | 23 mm | 26 mm |
| 6 | 20/519 | 40 mm | 40 mm | 35 mm | 0 mm | 31 mm | 24 mm |
| 7 | MNC 2175 | 31 mm | 27 mm | 23 mm | 28 mm | 20 mm | 28 mm |
| 8 | KNC 32 | 26 mm | 32 mm | 18 mm | 26 mm | 30 mm | 25 mm |
| 9 | C1805 | 28 mm | 28 mm | 24 mm | 27 mm | 26 mm | 25 mm |
| 10 | 330/1994 | 25 mm | 30 mm | 23 mm | 27 mm | 28 mm | 30 mm |
| 11 | C2325 | 32 mm | 32 mm | 20 mm | 31 mm | 23 mm | 29 mm |
| 12 | C2262 | 27 mm | 30 mm | 21 mm | 28 mm | 24 mm | 26 mm |
| 13 | C2264 | 27 mm | 33 mm | 20 mm | 32 mm | 24 mm | 28 mm |
| 14 | C2321 | 28 mm | 31 mm | 24 mm | 27 mm | 22 mm | 27 mm |
| 15 | C386 | 34 mm | 32 mm | 19 mm | 33 mm | 23 mm | 27 mm |
| 16 | C128 | 30 mm | 32 mm | 20 mm | 27 mm | 25 mm | 26 mm |
| 17 | C2554 | 32 mm | 30 mm | 22 mm | 32 mm | 29 mm | 33 mm |
Note: Resistant isolates are highlighted in red.
Table 2: The table shows drug sensitivity profiles (ZOI in mm) of isolates towards combination of drugs at 1:1 ratio.
| Zone of inhibition | |||||||
|---|---|---|---|---|---|---|---|
| S.No. | Sample | l+T | M+T | A+T | l+M | A+I | A+M |
| 1 | RPC 804 | 24 mm | 32 mm | 25 mm | 24 mm | 24 mm | 29 mm |
| 2 | C1654 | 24 mm | 30 mm | 17 mm | 27 mm | 24 mm | 28 mm |
| 3 | 37/1561 | 25 mm | 34 mm | 17 mm | 26 mm | 24 mm | 30 mm |
| 4 | RPC 937 | 27 mm | 32 mm | 23 mm | 27 mm | 16 mm | 30 mm |
| 5 | 64/316 | 26 mm | 30 mm | 23 mm | 28 mm | 17 mm | 32 mm |
| 6 | 20/519 | 40 mm | 40 mm | 39 mm | 31 mm | 27 mm | 31 mm |
| 7 | MNC 2175 | 30 mm | 36 mm | 26 mm | 29 mm | 20 mm | 31 mm |
| 8 | KNC 32 | 29 mm | 36 mm | 23 mm | 30 mm | 24 mm | 31 mm |
| 9 | C1805 | 30 mm | 32 mm | 22 mm | 28 mm | 23 mm | 31 mm |
| 10 | 330/1994 | 16 mm | 18 mm | 30 mm | 11 mm | 28 mm | 31 mm |
| 11 | C2325 | 40 mm | 36 mm | 30 mm | 30 mm | 27 mm | 27 mm |
| 12 | C2262 | 28 mm | 30 mm | 24 mm | 24 mm | 25 mm | 31 mm |
| 13 | C2264 | 27 mm | 29 mm | 25 mm | 24 mm | 26 mm | 28 mm |
| 14 | C2321 | 29 mm | 32 mm | 23 mm | 28 mm | 26 mm | 28 mm |
| 15 | C386 | 33 mm | 30 mm | 26 mm | 24 mm | 27 mm | 28 mm |
| 16 | C128 | 32 mm | 32 mm | 23 mm | 24 mm | 18 mm | 28 mm |
| 17 | C2554 | 34 mm | 31 mm | 27 mm | 26 mm | 27 mm | 28 mm |
Note: Resistant isolates are highlighted in red.
Table 3: The table shows drug sensitivity profiles (ZOI in mm) of isolates towards combination of drugs at 1:3 ratio.
| Zone of inhibition | |||||||
|---|---|---|---|---|---|---|---|
| S.No. | Sample | l+T | M+T | A+T | l+M | A+I | A+M |
| 1 | RPC 804 | 22 mm | 32 mm | 24 mm | 22 mm | 23 mm | 25 mm |
| 2 | C1654 | 21 mm | 34 mm | 16 mm | 23 mm | 20 mm | 19 mm |
| 3 | 37/1561 | 24 mm | 34 mm | 18 mm | 24 mm | 20 mm | 22 mm |
| 4 | RPC 937 | 27 mm | 34 mm | 20 mm | 22 mm | 18 mm | 20 mm |
| 5 | 64/316 | 27 mm | 34 mm | 19 mm | 22 mm | 19 mm | 19 mm |
| 6 | 20/519 | 40 mm | 40 mm | 32 mm | 32 mm | 29 mm | 29 mm |
| 7 | MNC 2175 | 29 mm | 33 mm | 25 mm | 26 mm | 20 mm | 27 mm |
| 8 | KNC 32 | 27 mm | 35 mm | 22 mm | 27 mm | 22 mm | 24 mm |
| 9 | C1805 | 28 mm | 31 mm | 20 mm | 26 mm | 25 mm | 21 mm |
| 10 | 330/1994 | 14 mm | 16 mm | 30 mm | 12 mm | 26 mm | 34 mm |
| 11 | C2325 | 30 mm | 35 mm | 29 mm | 28 mm | 29 mm | 28 mm |
| 12 | C2262 | 28 mm | 32 mm | 22 mm | 27 mm | 22 mm | 24 mm |
| 13 | C2264 | 30 mm | 31 mm | 21 mm | 28 mm | 23 mm | 25 mm |
| 14 | C2321 | 30 mm | 32 mm | 27 mm | 30 mm | 25 mm | 24 mm |
| 15 | C386 | 30 mm | 32 mm | 23 mm | 29 mm | 24 mm | 24 mm |
| 16 | C128 | 31 mm | 34 mm | 23 mm | 27 mm | 19 mm | 22 mm |
| 17 | C2554 | 32 mm | 34 mm | 23 mm | 27 mm | 21 mm | 26 mm |
Note: Resistant isolates are highlighted in red.
Table 4: The table shows drug sensitivity profiles (ZOI in mm) of isolates towards combination of drugs at 3:1 ratio.
Studies have shown the active and synergistic interaction of azoles with Terbinafine, Amphotericin, and Echinocandins (micafungin) against Candida isolates [40-43]. Another study has reported that a combination of Terbinafine and azole resulted in a fungicidal action against C. albicans, although the drugs were fungistatic when they were used individually [44]. Studies have also shown simultaneous inhibition of different fungal cell targets, giving synergism between Echinocandins (cell wall active) and Amphotericin. Antifungal drug combinations have also been used against planktonic and sessile cells of Candida albicans [45]. The combinations showed a higher cure rate, fewer relapses, and less nephrotoxicity than monotherapy with Amphotericin [46]. Similarly, Terbinafine has also shown synergistic effects due to their mechanism of actions, when used in combination with different drugs like azoles against Candida albicans and Candida glabrata infections [47,48]. Thus, it can be concluded that combination therapy could provide an effective alternative to monotherapy for patients with invasive infections that are difficult to treat due to multi-resistant species.
Statistical analysis of combinatorial drugs at different proportions vs. monotherapy
All the results obtained from drug sensitivity analysis were statistically analyzed as described in materials and methods using Prism. The drug combinations were compared to the efficacy by individual drugs along with the three stoichiometric ratios of a combination. Mean ± Std Dev of all the combinations and individual drugs are shown in Figures 3 and 4. For all the combinations, it was observed that the combinations were much better than monotherapy; however, no significant difference was observed between stoichiometric ratios except in the case of A+T and A+M combinations. In the A+T combination, the mean was significantly different in 1:3 proportions while insignificant in other proportions as well as to individual drugs. In the A+M combination, the mean for 1:1 and 1:3 was significantly different from the individual drug, and when used in the combination of 3:1, no difference was observed. All the p values obtained for different drug and drug combinations are summarized in Figure 5. A complete analysis of sensitivity of isolates towards different drug combinations is shown in Figure 4.
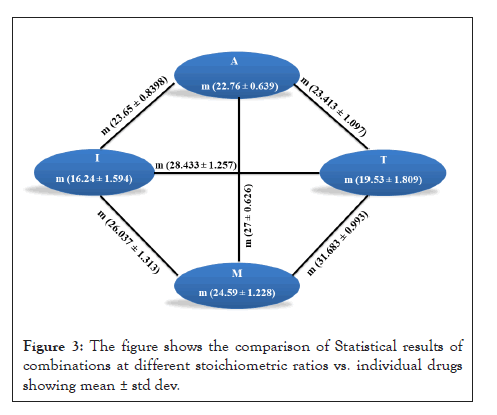
Figure 3: The figure shows the comparison of Statistical results of combinations at different stoichiometric ratios vs. individual drugs showing mean ± std dev.
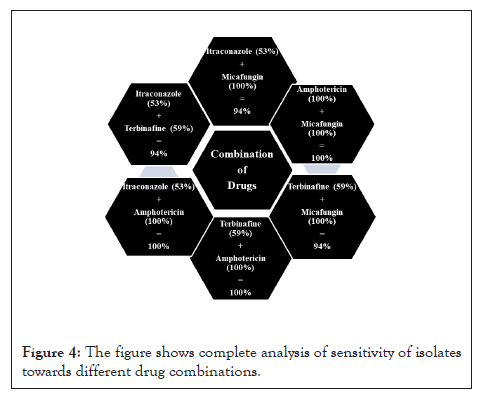
Figure 4: The figure shows complete analysis of sensitivity of isolates towards different drug combinations.
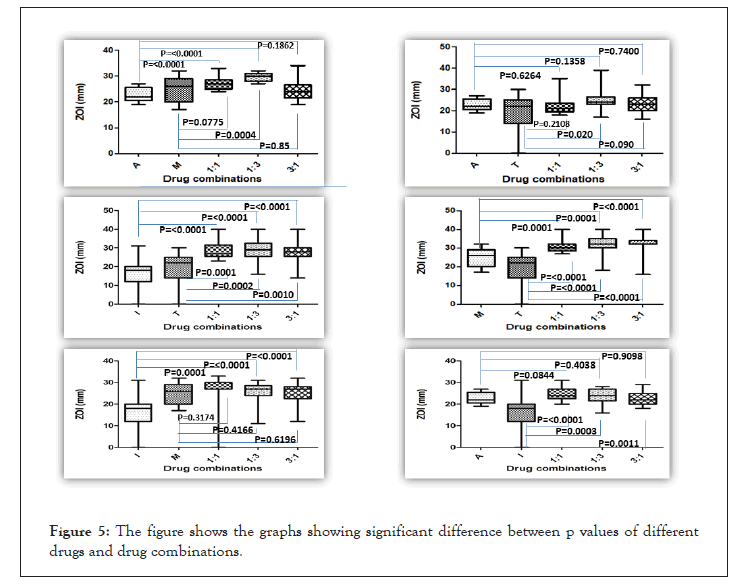
Figure 5: The figure shows the graphs showing significant difference between p values of different drugs and drug combinations.
The study is focused on the drug sensitivity analysis of Candida isolates towards various drugs and their combinations. Four different classes of drugs used for the study belonged to different classes with a different mechanism of action (Itraconazole, Amphotericin B, Micafungin and Terbinafine). Results have shown that the monotherapy drugs were not as efficacious. Thus, combinatorial drugs were prepared and analyzed at three different stoichiometric ratios. The results from this study showed that different isolates showed maximum sensitivities to the combinatorial drugs at all proportions. Most of the combinations were statistically significant from drug monotherapy except for Amphotericin B. No statistical significance was observed between the three different proportions of drug combinations. Combination therapy could provide an alternative to monotherapy for patients with invasive infections that are difficult to treat due to the emergence of resistance.
[Crossref]
[Crossref]
Citation: Agarwal S, Parida S, Jadoun B, Patel SN, Moolchandani S, Puniyani H, et al. (2022) In Vitro Potency of Combinatorial Antifungal Drugs towards Candida Isolates from Patients. Fungal Genom Biol. 12:203.
Received: 01-Nov-2022, Manuscript No. FGB-22-18879; Editor assigned: 03-Nov-2022, Pre QC No. FGB-22-18879 (PQ); Reviewed: 17-Nov-2022, QC No. FGB-22-18879; Revised: 24-Nov-2022, Manuscript No. FGB-22-18879 (R); Published: 02-Dec-2022 , DOI: 10.35248/2165-8056.22.12.203
Copyright: © 2022 Agarwal S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.