Medicinal & Aromatic Plants
Open Access
ISSN: 2167-0412
ISSN: 2167-0412
Research Article - (2015) Volume 4, Issue 1
Antioxidants play an important role in inhibiting the free radicals thereby provides protection against infection and degenerative diseases. Natural antioxidants are scavenging reactive oxygen species (ROS), they are preferred over synthetic antioxidants because of their safety over the synthetic antioxidant chemicals. Momordica dioica Roxb., have been used in Indian traditional medicine for various ailments like antiallergic, hepatoprotective , antihyperglycemic, nephroprotective and antimicrobial. In the present investigation sequentially extracts of various plant parts of M. dioica, and its tissue cultures were analyzed for their antioxidant potential. Standard protocols of scavenging effect on the DPPH free radical, Haemoglobin glycosylation Assay, Ferric Reducing Antioxidant Power, Nitric oxide and Lipid Peroxidation Assay were performed with some modifications. The fractionated extractives using different solvents analyzed for their antioxidant potential, the DCF of fruits with DPPH, MF of callus with HG and MF of stem with FRAP assays showed the highest AE. Elicitation increased the AE from 85 to173% in various treated samples. Effect of salt and Vitamin C gave significant blockage that increased with time treatment. Effect of various elicitors (sodium nitroprusside and salicylic acid), ascorbic acid and sodium chloride in different concentrations and time period on AE (antiradical efficiency) was also investigated. Results were expressed as AE, which is 1000/ IC50 in case of DPPH while in FRAP it was expressed as μM/l/g. M. dioica showed that this plant is a potent antioxidant agent. It is able to increase cellular survival by reducing intracellular lipid and protein oxidation levels and ROS scavengers.
Keywords: Antioxidant activity; Momordica dioica Roxb; Haemoglobin glycosylation; DPPH assay; Lipid peroxidation assay; FRAP
Oxidants are normal product of aerobic metabolism that can be produced at elevated rates under patho-physiological conditions. The continuous production of free radicals is balanced by an equivalent synthesis of antioxidants to combat the oxidative tissue damage. An imbalance between oxidants, reactive oxygen species (ROS), metabolite production and antioxidants in favour of the oxidants, potentially leading to damage are termed ‘oxidative stress’. Antioxidant defense involves several strategies, both enzymatic and non-enzymatic. A predominantly plant-based nutraceuticals possess antioxidant potential and exert multifaceted effect in correction of the metabolic imbalance are preferred over the synthetics [1].
Vitamins A, C and E are taken as standard antioxidants for comparison. The free radicals induce peroxidation of lipid membrane through LPO (lipid peroxidase) lead to deterioration and damage of membrane-bound enzymes during stress-ageing [2] and radiations [3]. NO (Nitric Oxide) is another important messenger molecule involved in both beneficial and detrimental patho-physiological processes in the body [4].
Elicitation originally used for defense mechanism is one of the most effective biotechnology strategies for improving the productivity of bioactive compounds in plants [5]. Elicitors besides enhancing plant defense, also elicit the specific bioactivities thereby increasing their bioefficacy.
Different antioxidant compounds act through different mechanism, no single method can fully evaluate the antioxidant activity. These assays have presented distinct challenges in evaluation of purified individual compounds, mixed extracts, fractions, herbs, supplementation of vitamins and other chemicals/medicines. Thus Optimization of right method/s of application and evaluation is the need of time [6].
Momordica dioica Roxb, belongs to the family Cucurbitaceae, commonly known as Teasle Gourd or “Spine Gourd” is native to India. It is used as vegetable. Tubers, fruits and roasted roots are used for intestinal ulcer, piles, snake bites, as antiseptic and antiinflammatory agents [7]. There are preliminary reports on antiallergic [8], hepatoprotective [9], antihyperglycemic [10], nephroprotective [11] and antimicrobial [12] activities of M. dioica.
Chemicals
Vitamin A, C and E, Sodium nitroprusside, Griess reagent, sulfanilamide, N-Naphthylethylenediamine dihydrochloride, Sodium nitrite, Trichloroacetic acid (TCA), Butylated hydroxytoluene, Thiobarbituric acid, L-methionine, Ethylene diamine tetra acetic acid, (EDTA), Nitroblue tetrazolium chloride, Riboflavin, H2O2, PVP, Pyrogallol , the stable free radical DPPH 3-(2-pyridyl)-5,6-bis (4-phenylsulfonic acid)-1,2,4-triazine (Ferrozine), TPTZ (2,4,6-tris (2pyridyl)-s-triazine) were obtained from Sigma (Sigma-Aldrich GmbH, Sternheim, Germany). Ammonium thiocyanate was purchased from Merck. Human blood for Haemoglobin glycosylation assay was obtained from Bhagwan Mahaveer Cancer Hospital and Research Centre, Jaipur India.
Tissue culture
Murashige and Skoog [13] medium (MS) was used for initiation and maintenance cultures of M. dioica. Nodal segments were surface sterilized with HgCl2 solution, rinsed thrice with sterile distilled water. The callus culture was established by using various treatment doses of hormones. These calli were maintained for about six months with frequent subculturings at time interval of 4-6 weeks. Growth index (GI) was calculated after 2, 4, 6 and 8 weeks of fresh sub culturing to record the growth pattern.
GI=Final wt. of the tissue – initial wt of the tissue/initial wt of the tissue.
Fractionation and determination of non-enzymatic antioxidant activity
All plant parts collected were cleaned and oven dried at 100°C for 10 min to deactivate the enzymes and then at 25°C till constant weight was achieved and then powdered. The voucher specimen of experimental plant was deposited in Herbarium (RUBL No. 20394*).
Screening and comparison of antioxidant activity of various plant parts and callus tissues were subjected to fractionated solvent extraction viz methanol (MeOH) fraction – MF; hexane fraction – HF; dichloromethane fraction – DCF and ethyl acetate fraction - EAF. All the fractions were subjected to determination of AE (Antiradical efficiency) using various established methods.
DPPH radical scavenging method
Plant extract (0.75 mL) at different concentrations ranging from 10 to 100 μg mL-1 was mixed with 1.5 mL of a DPPH methanolic solution (20 mg L-1). Pure methanol was taken as control and ascorbic acid (vitamin C), vitamins A and E were used as a reference compounds. The absorbance was measured at 517 nm after 20 min of reaction. The percent of DPPH decoloration of the sample was calculated according to the formula [14].
Decoloration%=[1 − (AbsSAMPLE/AbsCONTROL)] × 100
*RUBL Botanical Herbarium, Department of Botany, University of Rajasthan, Jaipur 302001
The decoloration was plotted against the sample extract concentration and a logarithmic regression curve was established in order to calculate the IC50. The results are expressed as antiradical efficiency (AE), which is 1000-fold inverse of the IC50 value AE=1000/ IC50, [15].
Haemoglobin glycosylation (HG) assay
1 mL each of glucose solution (2% w/v), blood sample (5 g/dl of Hb concentration, diluted with sodium citrate (4% w/v), gentamycin (0.2% v/v, dissolved in 0.01 M phosphate buffer) and each of the test sample (10-100 μg mL-1 extracts in distilled DMSO) was added and incubated in dark at room temperature for 3 days. Corresponding blank solution without sample and glucose were also incubated simultaneously. After incubation, the hemoglobin was measured in all the tubes. Vitamins A, C and E were used as a standard for comparison of the AE of test samples. The AE was calculated as follows:
Percent inhibition=(B – A)/B × 100
Where: B=Hb content (control without glucose – Control with glucose)
A=Hb content (control without glucose –Sample)
The percent inhibition was plotted against the sample extract concentration and a logarithmic regression curve was established in order to calculate the IC50 and results are expressed as AE [16]
Ferric reducing antioxidant power (FRAP) assay
The FRAP is another method of AE assay (18). The Fe++ interacts with TPTZ providing a strong absorbance at 593 nm [17,18].
Plant sample (1 g) were cut into small pieces and mashed with a cool mortar and pestle using quartz sand and 9 mL cool 0.1 M phosphate buffer was added. (pH 7.6, containing 0.1 mM EDTA). Each of the test mixture was filtered through a filter paper and centrifuged at 15,000 rpm for 10 min. The supernatant was used for the measurements of OD (optical density) at 593 nm after making up to 5 mL volume.
Calculation: The relative activities of samples were assessed by comparing their activities standard curve of ferrous sulphate. Ferrous sulphate was dissolved in distill water and different concentrations (100-1000 μM/L) was used for the measurement of OD. Standard FRAP reagent was used as blank.
Nitric oxide (NO) scavenging activity
For the quantification of total NO produced Griess reagent assay was used. 50 μL samples and reference compounds (250 μM mL-1, each) were diluted with equal amount of (50 μl) 10 mM SNP (sodium nitroprusside) solution and incubated at 25°C for 150 min. A regression curve was plotted taking 0.1 M sodium nitrite as standard ranged from 10 to 100 μL concentration in constant volume of 100 μL with distilled water. To each samples, reference compounds and 100 μL of standard Griess reagent (commercially available reagents A and B were combined in 1:1 ratio just before use) was added and absorbance was read at 542 nm. The nitrite concentration was calculated by referring to the absorbance of standard solutions of sodium nitrite. Results were expressed as percentage inhibition of nitrite production [19,20].
% Inhibition=(1 – SConc / CConc) × 100
Where SConc and CConc were the nitrite concentration of the sample and control, respectively.
Lipid peroxidation (LPO) assay
Each of 1 g fresh plant samples was homogenized with 25 mL of ethanol in pre chilled mortar and pestle and refrigerated centrifuged at 10000 rpm for 20 minutes at 4°C. The clear supernatant was taken as the enzyme extract. In one test tube, 1 mL of enzyme extract was added in 0.8 mL trichloro acetic acid (TCA; 20% w/v in water) and 0.2 mL of butylated hydroxytoluene (0.01% w/v in ethanol). In second test tube 1 mL of enzyme extract was added in 0.8 mL thiobarbituric acid (TBA; 0.5% w/v in 20% TCA) and 0.2 mL of butylated hydroxytoluene. These mixtures were incubated at 95°C for 25 min. The reaction was stopped by cooling in an ice bath for 15 min. Reaction tubes were centrifuged at 10000 rpm for 10 min and supernatants were subjected to absorbance data at 532 and 400 nm. The value for non-specific absorption at 600 nm was subtracted from value obtained after addition at 532 and 400 nm [21].
Elicitation
Fresh fruits and 6 months old maintained and established calli of M. dioica were taken and homogenized in pre chilled mortar and pestle with four treatment doses (0.025, 0.05, 0.075 and 0.1 mM) of abiotic elicitors SNP and salicylic acid (SA), respectively and kept in a flask. The flasks were incubated on reciprocal shaker with a constant rotation speed (125 rpm: 5 cm/stroke) for 6, 12, 18 and 24 h time treatment, separately. All the test samples after appropriate time of incubation were used for antioxidant enzyme estimations using various established methods as mentioned earlier.
Salt stress and ascorbic acid treatment
Fresh fruits and callus of M. dioica were taken and homogenized in pre chilled mortar and pestle with two treatment doses (50 and 100 mM) of sodium chloride (NaCl) and Vitamin C (0.05 and 0.1 mM), separately. The flasks were incubated on reciprocal shaker with a constant rotation speed (125 rpm: 5 cm/strokes) for 6, 12, 18 and 24 h, separately. After appropriate time of incubation, fruits were analyzed for antioxidant estimations as above.
Values are given as mean ± SEM (standard error of the mean) and were compared using one way ANOVA to judge the difference between various groups. Values of p<0.05 were considered statistically significant
The statistical error of mean was calculated by the following formula:-
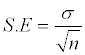
Where
σ=standard deviation
n=number of observations
The test of significance (t-test) was calculated by the following formula:
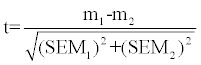
Where,
m1=mean of one set of values.
m2=mean of second set of values.
SEM1=standard error of the first set of values.
SEM2=standard error of the second set of values.
The probability ‘p’ for obtaining ‘t’ value of at least as great as the calculated one for a given number for the degree of freedom was found in the Fisher’s table.
The p-values were signified according to the following conventions.
P<0.05=difference was almost significant.
P<0.01=difference was significant.
P<0.001=difference was highly significant
Tissue culture
Unorganized callus was established from nodal segments inoculated on MS medium supplemented with BAP (benzyl adeno purine) and IBA (indole butyric acid) at treatment dose of 3 mgL-1, each after trying various hormonal treatment doses. Growth index (GI) was calculated at various time intervals of 2, 4, 6 and 8 weeks subculturings of fresh calli. The GI was found to be minimum in 2 weeks (0.85) and maximum (2.94) in 6 weeks old tissue. Thereafter, the GI steadily declined thus showing a characteristic sigmoid curve.
DPPH assay
The free radical (DPPH˙) scavenging activity expressed as AE ranged from 4.03 to 19.63. The dichloromethane fraction (DCF) of fruits showed the highest AE (19.63±0.79) followed by methanol fraction (MF) of callus tissue (19.51 ± 0.79) and hexane fraction (HF) of roots showed lowest AE (4.03 ± 0.85). The free radical (DPPH) scavenging activity of M. dioica fractions was found to be comparable to the well-known antioxidants such as vitamin C and E. Vitamin E showed highest free radical scavenging activity (54.17 ± 1.71) as compared to Vitamin A (47.16 ± 0.94) and Ascorbic acid (51.29 ± 1.22) (Table 1).
| Fractions | AE of different plant parts and callus | AE of Reference Compounds | ||||
|---|---|---|---|---|---|---|
| Fruits | Leaves | Stem | Roots | Callus | ||
| MF | 16.41 ± 0.98 | 18.82± 0.70 | 7.08± 0.88 | 4.63 ± 1.31 | 19.51 ± 0.79 | Vitamin A 47.16±0.94 |
| HF | 12.55 ± 1.13 | 14.09 ± 0.93 | 8.05 ± 0.76 | 4.03 ± 0.85 | 15.14 ± 0.82 | Vitamin C 51. 29 ± 1.22 |
| DCF | 19.63 ± 0.71 | 14.94 ± 0.97 | 9.96 ± 0.88 | 6.79 ± 0.82 | 16.34 ± 0.99 | Vitamin E 54.17 ± 1.71 |
| EAF | 11.24 ± 0.73 | 10.73 ± 0.77 | 5.31 ± 0.97 | 5.05 ± 1.08 | 16.24 ± 0.84 | Vitamin E 54.17 ± 1.71 |
Table 1: Antiradical Efficiency (AE) of M. dioica plant parts and callus cultures using DPPH radical scavenging activity.
Antioxidant activity and mechanisms are system dependent which vary with radical targets, individual v/s total antioxidant concentrations and solvent of extraction [22], presence of competing metals, pH and presence of oxygen [23] in various fractionated extracts and testing methods [24]. Therefore, different antioxidant compounds act through different mechanism and multiple methods can help to evaluate the antioxidant activity. Antioxidant reacts with DPPH free radical, as a result, electron becomes paired off and bleaching of the colour stochiometrically in MF depends on the number of electrons taken up. This diversity is due to the complexity of analysed substrates. In the present study some fractionated extracts of M. dioica plant parts and callus culture showed better AE than others confirms the above view. Present findings are in agreement with the reports of several workers who observed antioxidant potential of in vivo and in vitro grown tissue cultures of several other medicinal plants [25,26].
HG assay
AE ranged from 21.03 to 37.13 using HG assay in various plant parts and callus cultures. Highest activity (37.13 ± 0.74) was observed in MF of callus tissue and lowest (21.03 ± 0.75) in HF of roots. The free radical scavenging activity from HG assay of test plant was found to be comparable with well known antioxidants, such as ascorbic acid (vitamin C) and α-tocopherol (Vitamin E). Vitamin E showed the highest AE (76.19 ± 1.09) followed by vitamin C (74.96 ± 0.82) and Vitamin A (61.457 ± 1.34) (Table 2).
| Fractions | AE of different plant parts and callus | AE of Reference Compounds | ||||
|---|---|---|---|---|---|---|
| Fruits | Leaves | Stem | Roots | Callus | ||
| MF | 35.67 ± 0.68 | 26.67 ± 0.86 | 23.48 ± 1.13 | 23.23 ± 0.77 | 37.13 ± 0.74 | Vitamin A 61.457 ± 1.34 |
| HF | 32.55 ± 0.98 | 24.09 ± 0.79 | 25.05 ± 0.86 | 21.03 ± 0.75 | 35.14 ± 0.89 | Vitamin C 74.96 ± 0.82 |
| DCF | 29.63 ± 0.74 | 24.94 ± 0.74 | 26.96 ± 0.95 | 24.79 ± 1.10 | 32.34 ± 0.92 | Vitamin C 74.96 ± 0.82 |
| EAF | 31.24 ± 0.78 | 26.73 ± 0.81 | 25.31 ± 0.98 | 23.05 ± 0.86 | 33.24 ± 0.78 | Vitamin E 76.19 ± 1.09 |
Table 2: Antiradical Efficiency (AE) of M. dioica plant parts and callus cultures using Haemoglobin Glycosylation (HG) assay.
HG is an in vitro non-enzymatic method based on an oxidation reaction where an antioxidant is expected to inhibit the reaction [27]. Glyco-haemoglobin is formed throughout the circulatory life of RBC by the addition of glucose to the N-terminal of the haemoglobin β chain. This process which is non-enzymatic reflects the average exposure of haemoglobin to glucose over an extended period. The degree of haemo-glycosylation in vitro in the presence of different concentration of fraction can be measured. In the present study MF of various plant parts and callus tissue showed higher AE, when compared with standard reference compound (vitamin A, C, E). These results are in the agreement of reports [28] that sometimes extracts show synergic actions in comparison to the individual compounds due to their distinct antioxidant and pro-oxidant properties. Moreover, combinations of individual metabolite concentrations in the given extracts also play a complimentary or supplementary role in determining the bioactivity.
FRAP assay
AE ranged from 12.00 to 287. Highest activity was observed in MF of stem (287 ± 1.455) and lowest in MF of roots and HF of fruits. (19 ± 0.382) The values were based on standard regression curve of ferrous sulphate and calculated as mM/l/g (Table 3).
| Fractions | Fruits | Leaves | Stem | Roots | Callus |
|---|---|---|---|---|---|
| MF | 26 ± 0.57 | 108 ± 1.5 | 287 ± 1.455 | 16 ± 0.38 | 237 ± 0.39 |
| HF | 19 ± 0.382 | 105 ± 2.51 | 281 ± 2.685 | 21 ± 0.475 | 252 ± 1.99 |
| DCF | 23 ± 0.485 | 94 ± 1.0 | 250 ± 2.270 | 19 ± 0.989 | 245 ± 1.74 |
| EAF | 30 ± 0.230 | 120 ± 1.455 | 267 ± 2.375 | 21 ± 0.61 | 226 ± 1.85 |
Table 3: Antiradical Efficiency (AE) of M. dioica plant parts and callus cultures using FRAP assay.
FRAP assay is based on the reduction of Fe+3 to Fe+2 due to the action of antioxidants. Subsequently, the Fe+2 formed may interact with TPTZ providing a strong absorbance at 593 nm [18]. In the present study various sequential extracts of various plant parts and callus tissue showed higher AE when compared with standard regression curve of ferrous sulphate indicating superior AE of M. dioica extracts.
Nitric oxide (NO) scavenging activity
Fruits and callus analysed for NO, showed control value of 13.1 ± 0.45 in fruits and 16.67 ± 0.67 in callus.
Using SA as an elicitor NO gave maximum blockage (35.78 ± 0.57) in fruits after 12 h in 0.05 mM dose, which was 173.13% more than the control. Minimum NO activity (10.99 ± 0.56) was observed in 0.075 mM dose in fruits after 24 h, which was lower than the control. The NO activity increased till 0.05 mM concentration and then declined further.
Using SNP as an elicitor NO gave maximum blockage (26.78 ± 1.22) in fruits after 6 h in 0.025 mM, which was 104.42% more and minimum (10.08 ± 1.23) at 0.075 mM dose in callus after 24 h, which was lower than the control. The NO activity decreased on increasing treatment doses of elicitor.
Effect of Vitamin C (Aa) supplementation on NO in plant parts and callus showed gave highest blockage (34.30 ± 2.31) in fruits after 6 h at 0.05 mM, which was 161.83% higher and lowest (20.40 ± 1.12) at 0.1 mM dose in callus after 24 h, which was little higher than the control. The NO activity decreased with increasing concentration of treatment doses.
Effect of NaCl on NO showed maximum blockage (33.70 ± 1.60) in fruits after 12 h in 100 mM, which was more than 102.16% higher and minimum (11.44 ± 0.50) was 100 mM dose in callus, which was higher than the control (Table 4).
| Time (h) | Plant Parts | Control | Abiotic elicitors, ascorbic acidandsalt stress at various treatment doses (mM) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sodium Nitroprusside (SNP) | Salicylic Acid (SA) | Ascorbic Acid (Aa) | Sodium Chloride (NaCl) | |||||||||||
| 0.025 | 0.05 | 0.075 | 0.100 | 0.025 | 0.05 | 0.075 | 0.100 | 0.05 | 0.10 | 50 | 100 | |||
| 6 | Fruits | 13.1± 0.45 | 26.78 ± 1.22 | 18.57 ± 0.95 | 16.30 ± 1.22 | 13.66 ± 1.11 | 14.44 ± 1.32 | 28.17 ± 2.07 | 18.34 ± 1.30 | 13.20 ± 1.12 | 34.30 ± 2.31 | 27.60 ± 1.17 | 16.88 ± 1.16 | 25.64 ± 1.22 |
| 12 | Fruits | 22.60 ± 1.05 | 18.13 ± 0.50 | 11.21 ± 1.09 | 11.56 ± 0.75 | 20.54 ± 1.50 | 35.78 ± 0.57 | 17.51 ± 1.06 | 19.74 ± 1.34 | 26.11 ± 1.43 | 29.08 ± 1.22 | 20.71 ± 0.31 | 33.70 ± 1.60 | |
| 18 | Fruits | 21.11 ± 1.12 | 14.90 ± 0.53 | 11.20 ± 0.81 | 11.26 ± 1.08 | 16.06 ± 0.68 | 24.56 ± 1.25 | 16.40 ± 0.60 | 14.70 ± 1.13 | 29.46 ± 1.40 | 21.85 ± 1.12 | 23.90 ± 1.05 | 25.10 ± 1.41 | |
| 24 | Fruits | 20.10 ± 0.80 | 13.15 ± 0.80 | 12.11 ± 0.21 | 13.28 ± 0.98 | 14.14 ± 0.88 | 24.67 ± 1.51 | 10.99 ± 0.56 | 14.10 ± 1.14 | 25.16 ± 1.23 | 24.52 ± 1.46 | 21.21 ± 1.20 | 24.90 ± 1.20 | |
| Callus | 16.67 ± 0.67 | 20.12 ± 1.11 | 14.78 ± 0.80 | 10.08 ± 1.23 | 11.60 ± 0.07 | 17.23 ± 0.54 | 13.44 ± 0.77 | 11.22 ± 0.33 | 13.70 ± 0.30 | 25.11 ± 1.23 | 20.40 ± 1.12 | 13.74 ± 0.13 | 11.44 ± 0.50 | |
Table 4: Effect of various abiotic elicitors, ascorbic acid and salt stress on nitric oxide scavenging activity (%) of fruits and callus culture of M. dioica.
Plant cells respond to various biotic and abiotic elicitors by activating a wide array of reactions (viz ion fluxes across the plasma membrane, synthesis of ROS and phosphorylation and de-phosphorylation of proteins). These are all putative components of signal transduction pathways that lead to elicitor-induced defense responses, such as activation of defense genes and hypersensitive cell death [26,29]. It has been suggested that ROS alone cannot mediate a sufficient disease resistance response in plants, but in combination with NO can function synergistically to activate a better response [30]. Therefore, NO is a diffusible and bioactive signaling molecule [31,32]. The effect of abiotic elicitors (SA, SNP), Vitamin C and NaCl was determined on the fresh M. dioica fruits and in vitro grown six months old maintained callus cultures, which showed significant increase in various enzymatic activity after 6, 12, 18 and 24 h time intervals indicating M. dioica as a good antioxidant
The generation of NO is reported with SNP treatment [33]. NO plays a crucial role in the in cell suspension cultures and synthesis of secondary metabolites through various elicitors such as methyl jasmonate and salicylic acid [34-36]. NO is also a potent pleiotropic mediator of physiological processes such as smooth muscle relaxation, neuronal signaling, inhibition of platelet aggregation and regulation of cell mediated toxicity. All fresh test plant parts and callus inhibited NO in dose dependent manner. This may be due to antioxidants in the extract, which compete with ROS to react with NO.
In the present study the effect of an in vitro graded supply of SNP as NO producer and the relationship between NO and other elicitor responses, e.g., H2O2 (hydrogen peroxide) production, the activation of antioxidant defenses and effect on various other antioxidative enzymes was observed.. SNP and SA are the important group of pharmaceutical agents. The application of exogenous SA induces expression of defense related genes and provides partial protection against pathogen [37]. The NO scavenging activity in M. dioica decreased after increasing concentration of treatment doses of Vitamin C and SNP, separately and with SA it increased till 0.05 mM concentration and declined further.
Lipid peroxidation (LPO) assay
Fresh fruits and callus analysed for LPO content. In fruits the control value was 4.8 ± 0.98 while in callus it was 5.26 ± 0.099. Using SNP as an elicitor, maximum LPO content was observed (3.0 ± 0.72) after 6 h in 0.025 mM and minimum (-0.1 ± 0.02) at 0.1 mM dose after 18 h in fruits. When time period of supplementation dose was increased, the LPO content was gradually declined.
Using SA as an elicitor, highest LPO content was observed in fruits (8.91 ± 0.80) after 24 h in 0.025 mM, which was 85.63% higher as compared to control value and lowest (0.1 ± 0.03) at 0.1 mM dose after 18 h. The LPO content increased with increasing time period. On supplementation of vitamin C, LPO content decreased with increasing various treatment doses in callus whereas in fruits, it increased. Using NaCl as elicitor, LPO content increased in fruits with increasing concentration of treatment doses whereas in callus, it was decreased (Table 5).
| Time(h) | Plant Parts | Control | Abiotic elicitors, ascorbic acid and salt stress at various treatment doses (mM) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sodium Nitroprusside (SNP) | Salicylic Acid (SA) | Ascorbic Acid (Aa) | Sodium Chloride (NaCl) | |||||||||||
| 0.025 | 0.05 | 0.075 | 0.100 | 0.025 | 0.05 | 0.075 | 0.100 | 0.05 | 0.10 | 50 | 100 | |||
| 6 | Fruits | 4.8± .98 | 3.0± 0.72 | 1.2 ± 0.60 | 1.2 ± 1.33 | 0.1 ± 2.33 | 5.98 ± 1.33 | 2.8 ± 1.45 | 1.1 ± 0.81 | -1.2 ± 0.34 | 0.22 ± 0.22 | 0.60 ± 0.14 | 1.33 ± 0.22 | 1.90 ± 0.66 |
| 12 | Fruits | 2.67 ± 1.22 | 2.4 ± 0.45 | -1.1 ± 0.03 | -0.1 ± 0.15 | 6.40 ± 1.42 | 3.9 ± 0.50 | 1.1 ± 0.80 | -0.1 ± 0.33 | -0.04 ± 0.80 | 0.50 ± 0.10 | 0.25 ± 0.04 | 1.33 ± 0.45 | |
| 18 | Fruits | 2.42 ± 0.80 | 2.1 ± 0.90 | -0.4 ± 0.03 | -0.1 ± 0.02 | 6.5 ± 0.99 | 3.8 ± 0.80 | 0.40 ± 0.02 | -0.1 ± 0.03 | -0.09 ± 0.15 | 0.58 ± 0.10 | 0.23 ± 0.09 | 3.24 ± 0.77 | |
| 24 | Fruits | 2.20 ± 0.90 | 1.2 ± 0.80 | -0.2 ± 0.03 | -0.2 ± 0.32 | 8.91 ± 0.80 | 4.2 ± 0.77 | 0.35 ± 0.56 | -1.2 ± 0.05 | -0.05 ± 0.34 | 0.34 ± 0.43 | 0.20 ± 0.53 | 2.10 ± 0.10 | |
| Callus | 5.26 ± 0.099 | 2.504 ± 1.04 | 2.70 ± 0.87 | 0.30 ± 0.04 | 1.05 ± 0.86 | 3.90 ± 0.90 | 4.11± 0.80 | 2.40 ± 0.45 | 1.14 ± 0.36 | 0.76 ± 0.045 | 0.55 ± 0.075 | 2.30 ± 0.080 | 1.75 ± 0.075 | |
Table 5: Effect of various abiotic elicitors, ascorbic acid and salt stress on lipid peroxidation content (n mol ml-1) of fruits and callus culture of M. dioica.
Whereas the LPO content gradually declined with time and increased doses of SNP. Taking Vitamin C treatment, LPO content decreased with increasing treatment doses in callus and in fruits it was increased, whereas NaCl treatment increased LPO in fruits with increasing treatment doses and it decreased in callus. The above findings are supported by earlier finding [38] on MF of pod extract of M. oleifera that exhibited significant reduction in free radical scavenging by DPPH free radical, superoxide radical, H2O2 and NO production. Use of elicitors for evaluation AE is the first report in the present study. SA is a natural and hormone-like signal molecule for the activation of plant defenses, and regulates a large variety of physiological processes in plants [39]. SA and SNP are important group of pharmaceutical agents act as abiotic elicitor [40] and fungal elicitors are biotic elicitors. They are involved in several processes in plants that has important signaling role in plant defense mechanism against pathogens and formation of bioactive compounds [41]. Recently, Sharma and Singh [42] have found antioxoidant status of M. dioica on liver, pancreas and kidney cells of diabetic rats.
From the present study, it can be concluded that M. dioica is a good source of antioxidant. The fractionated extractives using different solvents analyzed for their antioxidant potential, the DCF of fruits with DPPH, MF of callus with HG and MF of stem with FRAP assays showed the highest AE. Elicitation increased the AE from 85 to173% in various treated samples. Effect of salt and Vitamin C give significant blockage that increased with time treatment that increased with time upto 18 h thereafter declined. It was observed that AE decreased with increase in concentration of elicitors along with time treatment. Overall non enzymatic activity initially increased and later decreased when measured by various assays. Evaluation of AE of these isolates of M. dioica showed that this plant is a potent antioxidant agent. It seems to be able to increase cellular survival by reducing intracellular lipid and protein oxidation levels, or either ROS scavengers. Further, it should be more promising to investigate the underlying molecular mechanism/s associated and also long term residual toxicity studies, if any, in different animal models as well as on human diabetic patients to ascertain its therapeutic ventures.
Authors are thankful to UGC New Delhi, India for funding and fellowship to one of the author Mr. Manas Mathur.
The authors declare that there are no conflicts of interests.