

Research Article - (2022)Volume 6, Issue 3
Aim: This research evaluated the effectiveness of the otological solution based on cajá leaves (Spondias mombin L. -SM) as a therapeutic alternative in the treatment of bacterial external otitis in dogs.
Methodology and results: For that, 20 dogs, without sex, breed and defined ages were divided into four groups, the first group used 2% SM diluted in glycerin and applied 24/24 h, the second group used 2% SM diluted in glycerin and applied 12/12 h, the third group as a positive control ciprofloxacin at 3.5% and the fourth group with SM at 20% applied 12/12 h. After 21 days of treatment, the alternative antimicrobial SM at 2% diluted in glycerin and applied for 12/12 h showed a reduction in the Colony Forming Unit (CFU) with no statistical difference from ciproflaxacin, no toxicological changes on the parameters of morphology, viability and epithelial cell activity.
Conclusion: The use of Spondia mombin reduced the number of bacterials and the clinical signs evaluated, proving its effectiveness as a treatment for otitis in dogs.
Spondia mombin; Medicinal plants; Antibacterial; Canine otitis; Counting; Toxicity; Effectiveness
Brazil has the richest plant biodiversity in the world; it is home to the Caatinga biome that houses numerous plants with medicinal potential. Herbal medicines are recognized for their effectiveness in treating illnesses while having few side effects, low cost, and high acceptability by society. Thus, the use of these products is an alternative to commonly used allopathic medicine while minimizing social, economic, cultural, and environmental problems [1].
Spondias mombin L. (SM) is a fruit tree, that reaches heights of 30 m, with trunks around 60 cm-75 cm wide, deciduous leaves around 9 cm-11 cm in size that have 5–11 leaflets, and small white flowers arranged in bunches at the end of the branch. The fruits, called drupes, are yellowish and can be around 2-4 cm long and 1.5 cm–2 cm wide. In northeastern Brazil, it is popularly known as cajazeira or caja [2]. The plant has active principles including flavonoids, flavonols, and condensed tannins that have various benefits such as antimicrobial properties [3].
Bacterial resistance to allopathic antimicrobials is becoming a pressing issue for medical professionals. Resistance is responsible for increased treatment costs and higher mortality rates due to untreatable infections; it is now a challenge for clinical management [4]. The use of herbal medicines such as SM that is already widely used by several communities may be a suitable alternative to treat diseases caused by multidrug-resistant bacteria.
Otitis is a common veterinary disease; it is characterized by inflammation and infection in the ear caused by microorganisms such as Pseudomonas aeruginosa. The number of bacterial strains that are multidrug resistant and that are encountered in the daily routine of veterinary medicine is on the rise due to genes in plasmids [5].
This study aimed to evaluate the antimicrobial efficacy of an otological solution made from caja leaves for the treatment of bacterial external otitis in dogs, as this is a promising antimicrobial alternative according to Dantas, et al. and Camila, et al. [1,2].
Experiments locations
The experiment, including the production of the SM decoction and the processing of biological samples, was conducted at the Veterinary Microbiology Laboratory (LAMIV), and the cytotoxicity evaluation was carried out at the Laboratory of Animal Biotechnology (LBA) of the Federal Rural University of the Semi- Arid (UFERSA), Mossoró, RN, Brazil.
Ethical considerations
The study was approved by the Ethics Committee on the Use of Animals (CEUA), from the UFERSA, number 01/2020, protocol no. 23091.014195/2019-78.
Decoction collection and production
The SM leaves were collected at 7:00 a.m. at Mossoro, RN (coordinates-5.200276184508256,-37.32056735242512). The plant species was identified in the herbarium Dárdaro of Andrade Lima and catalogued under number 13953. The solution was produced under decoction at a concentration of 20% for 10 min and then diluted to 2% in a glycerin solution.
Identification of bacteria and antibiogram
For the identification of bacteria during treatments, otological secretions were placed in Petri dishes containing 5% defibrinated sheep blood and MacConkey agar (Himedia, Mumbai, India) and incubated at 37˚C for 24–48 h; cultures were then subjected to identification as previously described (MacFaddin, 2000). The sensitivity of bacteria to antimicrobials Amoxicillin+Clavulanic Acid (AMC), Amikacin (AMI), Ampicillin (AMP), Aztreonam (ATM), Ceftriaxone (CRO), Cefepime (CPM), Cefoxitin (CFO), Cephalothin (CFL), Ceftazidime (CAZ), Ciprofloxacin (CIP), Chloramphenicol (CLO), Gentamicin (GEN), Piperacillin+Tazobactam (PIT), Tetracycline (TET), Sulfamethoxazole+Trimethoprim (SUT) (Laborclin) was performed according to the diffusion technique.
In vivo testing on dogs
The in vivo antibacterial potential of the otological solution was verified in 15 adult dogs with external bacterial otitis, through the quantification of bacteria under the action of SM-based decoction in three groups (5 dogs each), SM 2% diluted in glycerin and applied 24/24 h (SM 2% GLY24/24 h), SM 2% diluted in glycerin and applied 12/12 h (SM 2% GLY12/12 h), SM 20% applied 12/12 h (SM 20% 12/12 h), and 5 adult dogs used ciprofloxacin (CIPRO 3.5% 12/12 h) as a positive control, at days 7, 14, and 21 after initiation of treatment. Treatments consisted of 7 drops (per application) of the corresponding solution instilled into each animal.
Otological secrections were recovered from each dog, homogenized in a tube containing 2 mL of distilled water, and submitted to dilutions of 101 to 104. Dilution samples (1 mL each) were placed in Petri count plates in agar, in duplicates, incubated in a bacteriological incubator (Fanem, Sao Paulo, Brazil) at 37˚C for 24 h, and quantified by the plate counting technique [6].
Cytotoxicity of Spondia mombin: To evaluate cytotoxicity, skin fragments (9.0 mm3) were cultured in vitro in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% bovine fetal serum and 2% of an antibiotic-antimycotic solution (10,000 units/ mL penicillin, 10,000 units/mL streptomycin, and 25 μg/mL amphotericin, Gibco BRL, Carlsbad, CA, USA) at 38.5°C and 5% CO2. Epithelial cells cultivated in the 3rd passage were used for toxicity analysis at 80% confluence and at a concentration of 5.0 × 104 cells/mL. These cells were then divided into four groups and incubated for 12 h; cell groups were either grown in the absence of the SM decoction (SM 0) or in the presence of 20% SM decoction (SM 20), 2% SM decoction (SM 2), or ciprofloxacin 3.5% (CIPRO 3.5%). After the incubation period, cell morphology was evaluated using an inverted microscope (Nikon TS100, Tokyo, Japan). In addition, cell viability was evaluated using trypan blue (0.4%), where blue-colored cells were considered non-viable and colorless cells were considered viable. Briefly, after trypsinization, cells were incubated with trypan blue and counted in a Neubauer chamber (Kasvi), and the viability rate was calculated according to the formula: (number of living cells/total number of counted cells) × 100. For the evaluation of cell metabolic activity, an assay of 3-(4,5-dimethyl, 2-thiazolil)-2,5-diphenyl-2, il-tetrazolic (MTT) was used. Briefly, the cells were incubated with 5 mg/mL MTT for 3 h. After this period, the MTT solution was removed and 1.0 mL of Dimethyl Sulfoxide (DMSO) was added. The absorbance of the samples was measured using a spectrophotometer (Shimadzu® UVmini- 1240, Kyoto, Japan) at 595 nm.
Observations of clinical signs in animals: Clinical signs, including hyperemia, hyperpigmentation, hyperkeratosis, flaking, presence of secretion, pain, and pruritus, were evaluated using a checklist on days 0, 7, 14, and 21; they were assigned scores varying from +1 to +5 to determine the efficacy of the solutions [1].
Statistical analysis
Descriptive statistics were performed with the number and percentage of species and bacterial resistance. The results from the quantification of bacteria, cytotoxicity, and clinical signs were submitted to an Analysis of Variance (ANOVA) and the significance was found between the treatments. In addition, the mean comparison test (Tukey at 5% probability) was applied using the statistical program SISVAR 5.6. For cytotoxicity, the data were expressed as the mean ± standard error of the four repetitions and analyzed using Graphpad software (Graph-Pad Software Incorporated, La Jolla, CA, USA). All results were verified for normality using the Shapiro-Wilk test and for homoscedasticity using the Levene test. Those data that did not present a normal distribution were transformed using arc sine.
Identification and antibiogram
In total, 33 bacterial strains were isolated. The results regarding the etiology of otitis cases, as well as the frequencies of these isolations, are presented in Table 1. Among the 33 bacterial strains, 32 (97%) showed resistance to at least one antimicrobial and 9 (27%) were resistant to three or more different antimicrobials, according to results analyzed in the table of diameters of halos of the Brazilian Committee for Testing of Antimicrobial Susceptibility (BrCAST), as described in Table 2. From these, 32 (97%) strains showed resistance to amoxicillin + clavulanic acid (AMC) and ceftazidime (CAZ), followed by 30 (91%) strains showing resistance to ampicillin (AMP), cefepime (CPM), and aztreonam (ATM). The lowest index was for the antimicrobial ciprofloxacin (CIP) to which only 9 (27%) bacterial strains showed resistance. The genera of the isolated bacteria and their antimicrobial resistance profiles are described in Table 3.
| Microorganisms | No. of isolates | Percentage |
|---|---|---|
| Citrobacter freundii | 1 | 4% |
| Enterococcus spp. | 1 | 4% |
| Pantoea agglomerans | 1 | 4% |
| Stomatococcus spp. | 1 | 4% |
| Proteus spp. | 2 | 6.1% |
| Enterobacter spp. | 3 | 9.1% |
| Streptococcus spp. | 8 | 24.3% |
| Staphylococcus spp. | 16 | 44.5% |
Table 1 : Number and percentage of microorganisms isolated from dogs with otitis treated with Spondias mombin L. decoction at 2% or 20% or with ciprofloxacin at 3.5%.
| Antimicrobials | No. of resistant strains | % of resistant strains |
|---|---|---|
| Ciprofloxacin (CIP) | 9 | 27% |
| Chloramphenicol (CLO) | 11 | 33% |
| Ceftriaxone (CRO) | 12 | 36% |
| Amikacin (AMI) | 13 | 39% |
| Cephalothin (CFL) | 13 | 39% |
| Gentamicin (GEN) | 13 | 39% |
| Sulfamethoxazole+Trimethoprim (SUT) | 13 | 39% |
| Cefoxitin (CFO) | 16 | 48% |
| Tetracycline (TET) | 18 | 55% |
| Piperacillin+Tazobactam (PIT) | 23 | 70% |
| Ampicillin (AMP) | 30 | 91% |
| Aztreonam (ATM) | 30 | 91% |
| Cefepime (CPM) | 30 | 91% |
| Amoxicillin+Clavulanic Acid (AMC) | 32 | 97% |
| Ceftazidime (CAZ) | 32 | 97% |
Table 2 : Number and percentage of antimicrobial resistances among bacteria isolated from dogs with otitis treated with Spondias mombin L. decoction at 2% or 20% or with ciprofloxacin at 3.5%.
| Bacteria | Resistance profile |
|---|---|
| Citrobacter freundii | AMC, CIP, GEN, AMP, CPM, CRO, CFO, PIT, ATM, CAZ |
| Enterobacter spp. | AMC, SUT, CIP, CFL, GEN, AMP, AMI, CPM, TET, CRO, CFO, PIT, ATM, CLO, CAZ |
| Enterococcus spp. | AMC, AMP, CPM, ATM, CAZ |
| Pantoea agglomerans | AMC, CFL, AMP, CPM, CRO, CFO, PIT, CLO, CAZ |
| Proteus spp. | AMC, SUT, CFL, GEN, AMP, AMI, CPM, TET, CRO, CFO, PIT, ATM, CLO, CAZ |
| Staphylococcus spp. | SUT, CIP, CRO, CFL, AMI, GEN, TET, CFO, PIT, CPM, AMP, ATM, CLO, CAZ |
| Stomatococcus spp. | AMC, SUT, CIP, CFL, GEN, AMP, AMI, CPM, TET, CRO, CFO, PIT, ATM, CLO, CAZ |
| Streptococcus spp. | AMC, SUT, CIP, CFL, GEN, AMP, AMI, CPM, TET, CRO, CFO, PIT, ATM, CLO, CAZ |
Table 3 : Genera of bacteria isolated and their antimicrobial.
Spondia mombim performance in dogs
The results of the microbial counts of the different groups at 0, 7, 14, and 21 days are shown in Figure 1. There was a relation between the total number of Colony-forming Units (CFUs) and the number of days of treatment. A linear behavior was verified for treatments in the SM 2% GLY24/24 h and SM 2% GLY12/12 h groups; such similar behaviors showed a tendency to reduce the number of CFUs over the days of application. The means were adjusted to a quadratic model.
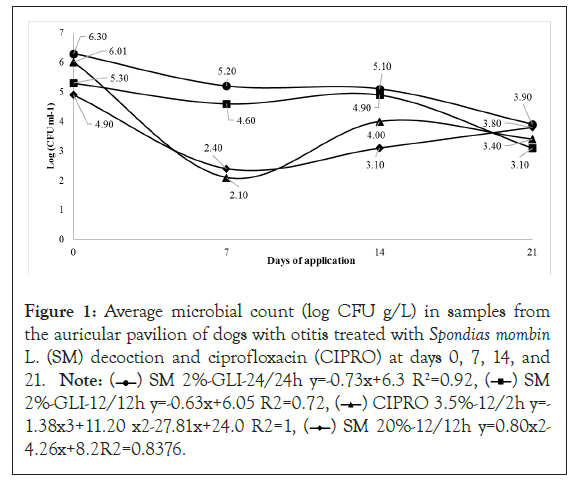
Figure 1: Average microbial count (log CFU g/L) in samples from
the auricular pavilion of dogs with otitis treated with Spondias mombin L. (SM) decoction and ciprofloxacin (CIPRO) at days 0, 7, 14, and
21. Note: ( ) SM 2%-GLI-24/24h y=-0.73x+6.3 R2=0.92, (
) SM 2%-GLI-24/24h y=-0.73x+6.3 R2=0.92, ( ) SM
2%-GLI-12/12h y=-0.63x+6.05 R2=0.72, (
) SM
2%-GLI-12/12h y=-0.63x+6.05 R2=0.72, ( ) CIPRO 3.5%-12/2h y=-
1.38x3+11.20 x2-27.81x+24.0 R2=1, (
) CIPRO 3.5%-12/2h y=-
1.38x3+11.20 x2-27.81x+24.0 R2=1, ( ) SM 20%-12/12h y=0.80x2-
4.26x+8.2R2=0.8376.
) SM 20%-12/12h y=0.80x2-
4.26x+8.2R2=0.8376.
Cytotoxicity of Spondia mombin: In vitro cytotoxicological analyses showed no signs of toxicological changes in morphology after treating cells with SM decoction at concentrations of 2% (SM 2% GLY) and 20% (SM 20%) (Figure 2). Additionally, viability parameters were similar among cells treated with SM decoction (either SM 2% GLY or SM 20%), a ciprofloxacin solution at 3.5% (CIPRO 3.5), or distilled water (control group, CTR 0) (Table 4).

Figure 2: Morphological evaluation of epithelial cells exposed to 20% SM decoction 2% SM decoction, and ciprofloxacin 3.5%. A) Negative control, B) 20% SM decoction, C) 2% SM decoction, D) ciprofloxacin 3.5%. Scale bar=100 μm; 100X magnification.
| Groups | Cell viability (%) | P compared to the control |
|---|---|---|
| CTR 0 | 67.3 ± 11.4a | -- |
| SM 20 | 62.0 ± 5.7a | 0.8553 |
| SM 2 | 77.8 ± 9.1a | 0.4322 |
| CIPRO 3.5 | 76.1 ± 4.9a | 0.5973 |
NOTE: aThe results are same |
||
Table 4 : Viability of epithelial cells grown in vitro with SM decoction at different concentrations (2% and 20%) and ciprofloxacin 3.5%. Values are expressed as mean ± standard deviation (SD) CTR 0: Negative control; SM 2: Spondias mombin 2% extract; SM 20: Spondias mombin 20% extract; CIPRO 3.5: Ciprofloxacin 3.5%.
There was no difference in the percentage of metabolic activity (Table 5) between the cells cultivated in the presence of SM decoction at both concentrations tested (20% and 2%). However, the percentage of metabolic activity of cells grown in the presence of CIPRO at 3.5% differed from that of the negative control (p<0.05).
| Groups | Metabolic activity (%) | P compared to the control |
|---|---|---|
| CTR 0 | 100.0 ± 1.5a | -- |
| SM 20 | 100.0 ± 4.7a | 0.9993 |
| SM 2 | 94.1 ± 4.9a | 0.1112 |
| CIPRO 3.5 | 6.8 ± 1.8b | 0.0001 |
| NOTE: a,bDifferent superscript letters indicate statistically significant differences (p<0.05) | ||
Table 5: Metabolic activity of epithelial cells grown in vitro with Spondias mombin decoction at different concentrations (20% and 2%) and ciprofloxacin at 3.5%. Values are expressed as mean ± standard deviation (SD). a,b: Different superscript letters indicate statistically significant differences (p<0.05). CTR 0: Negative control; SM 2: Spondias mombin 2% decoction; SM 20: Spondias mombin 20% decoction; CIPRO 3.5: Ciprofloxacin 3.5.
Observations of clinical signs in animals: The results of the macroscopic analysis of the clinical signs of otitis during the 21 days of treatment are described in Figure 3. The scores for pruritus, pain, hyperpigmentation, flaking, erythema, and exudate in the SM 2% GLY12/12 h and SM 20% 12/12 h groups are similar to those of the positive control CIPRO group (p<0.05).
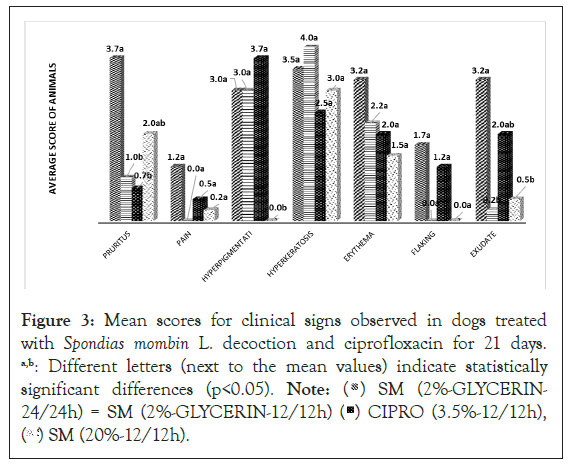
Figure 3: Mean scores for clinical signs observed in dogs treated
with Spondias mombin L. decoction and ciprofloxacin for 21 days.
a,b: Different letters (next to the mean values) indicate statistically
significant differences (p<0.05). Note: ( ) SM (2%-GLYCERIN-
24/24h) = SM (2%-GLYCERIN-12/12h) (
) SM (2%-GLYCERIN-
24/24h) = SM (2%-GLYCERIN-12/12h) ( ) CIPRO (3.5%-12/12h),
(
) CIPRO (3.5%-12/12h),
( ) SM (20%-12/12h).
) SM (20%-12/12h).
Identification and antimicrobial activity
Staphylococcus spp. had the highest incidence because they are common members of the ear canal microbiome; however, they may behave as opportunistic bacteria under certain host conditions [7]. Thus, a therapy with MS, which presents tannins, can be an alternative form of treatment for these bacteria, promoting binding with metal ions and forming a protective epithelial biofilm [3].
Streptococcus spp. is also considered as causative agents of otitis; they play a role as pathogens and favor the development of infections. Bioactive compounds present in SM such as flavonoids [8] may act as inhibitors of these microorganisms, representing the possibility of combating antimicrobial-resistant bacteria commonly found in veterinary medicine.
Identified enterobacterial strains (Pantoea agglomerans, Proteus spp., Enterobacter spp., Citrobacter freundii, Enterococcus spp., and Stomatococcus spp.) may have been acquired from the environment (from raw food, soil, water sources, plants, or wastewater), other animals (from saliva, urine, and feces), or humans [4,9,10]. These bacteria commonly cause infections in animals and exhibit antimicrobial resistance (mainly to penicillin and aminoglycosides), which is a problem in veterinary clinics. Thus, bioactive compounds such as catechins, flavanones, xanthones, and steroids present in SM leaves may be used for the production of potential drugs against these microorganisms [3].
Resistance to clavulanic acid+amoxicillin probably occurred due to genetic factors such as the presence of cassettes in DNA (SCCmec) in bacteria such as Staphylococcus spp [11].
These results showed similarity with reports on the emergence of multidrug-resistant bacteria [12] and are probably influenced by the lack of regulation of broad-spectrum antimicrobials in veterinary clinics, which further adds pressure on the selection of skin's bacteria.
Six multidrug-resistant genres were identified in the present study: Citrobacter freundii, Enterobacter spp., Proteus spp., Staphylococcus spp., Stomatococcus spp., and Streptococcus spp. [13] stated that a microorganism is considered multi-resistant when it is resistant to three distinct classes of antimicrobials simultaneously due to factors associated with plasmids. Staphylococcus spp. may have strong epithelial extracellular structures, form biofilms, and present mecA-associated resistance (mecA is a gene found in bacterial cells which allows them to be resistant to antibiotics such as methicillin, penicillin and other penicillin-like antibiotics. The bacteria strain most commonly known to carry mecA is methicillinresistant Staphylococcus aureus (MRSA), all of which hinder the antimicrobial activity of drugs, especially of β-lactam drugs (penicillin, cephalosporins, carbapenems, and monobactams), causing persistent infections [14].In a study by Davis, et al. [15], from the 11 samples of the genus Staphylococcus spp. obtained from dogs, 10 had the same resistance profile, being resistant to eight different antimicrobials, including ampicillin and ciprofloxacin.
The resistances of the Citrobacter freundii strain may indicate the presence of Amp-C β-lactamase (Amp-C), broad-spectrum β-lactamase, and extended-spectrum β-lactamase (ESBL) enzymes [4] Enterococcus spp. strains may be resistant to β-lactams through the PBP4 and PBP5 genes, to aminoglycosides due to the change in the transport of the drug into the microbial cell, and to glycopeptides due to the presence of operons modifying the antimicrobial binding site [16]. Strains of Streptococcus spp. may have PBPs (1a, 1b, 2a, 2b, 2x, and 3) that alter the affinity of binding to antimicrobials [17]; they are mainly acquired by DNA transfer (by transformation). The ability to modulate the mechanisms of resistance to β-lactams, such as the cephalosporinase of the type ß-lactamase AmpC and β-lactamases TEM-24, SHV, or CTX-M [18] may be present in strains of Enterobacter spp. Proteus spp. are usually resistant to β-lactams due to their capacity of producing metallo- β-lactamases and carbapenemases, mediated mainly by genetic elements in plasmids [19].
Spondia mombim testing in dogs
There was a general decrease in CFUs after 7 days of application of all treatments, with averages for SM 2%-GLY24/24 h, SM 2% GLY12/12 h, SM 20% 12/12 h, and CIPRO 3.5% 12/12 h groups at 5.20, 4.60, 2.10, and 2.40 log CFU.ml-1, respectively. At 14 days, the number of bacteria recovered after treatment with decoction SM 20% 12/12 h (3.10) was smaller than that for the CIPRO group (4.00) (p<0.05), and those for the SM 2% GLY12/12 h (4.60) and the CIPRO (4.00) groups were similar. Although the mean number of bacteria recovered from the SM 2% GLY24/24 h group (5.40) was the highest, there was no statistically significant difference between this and the CIPRO group. The increase in the number of bacteria at 14 days, compared to that in day 7, may be due to antimicrobial resistance of gram-negative bacteria, as described by [4]. At 21 days, the used of all alternative antimicrobials gave similar results, with no statistically significant differences compared to CIPRO. The decrease in the number of bacteria in these groups over time probably occurred because of the presence of glycerin. This compound probably allowed greater permanence of SM ingredients [20], and supported a gradual and prolonged performance of active principles, such as geranine and galoylgeranine bioactives, two different ellagitannins, chlorogenic acid, 2-caffeoyl acid, salicylic acid, triterpene 3β-urs-12-en-3-il(9z)- hexadec-9-enoate [21], and gaelic tannins, that have medicinal properties that kill gram-positive bacteria. Thus, the use of an herbal medicine with SM can be indicated as therapeutic alternative containing active ingredients that act significantly in the inhibition of microorganisms, as verified by [2]. Also observing the efficacy of SM decoction in inhibiting strains of Staphylococcus coagulasenegative as well as in healing surgical wounds of procedures such as salpingo-oophorectomy and orchiectomy [1].
Cytotoxicity of Spondia mombin: The utility of Spondias mombin L. decoction in the treatment of diseases such as otitis confirm the observations by [5]. Noteworthy, no biochemical, hematological, or behavioral changes were observed in rats treated with up to 2000 mg/kg of Spondias mombin × Spondias tuberosa decoction. The results in vitro are promising regarding plant cytotoxicity; they confirm the results of Abiodun, et al. [3], who reported that SM decoction does not promote cellular toxicity because it contains phenolic compounds (such as capsaicin and dihydrocapsaicin). Moreover, SM decoction may have cellular antioxidant capacity due to the presence of flavonoids [22]. The results from the CIPRO group can be justified according to Guimarães, et al. [23], who confirmed that these drugs inhibit cellular topoisomerases of bacteria such as Pseudomonas aeruginosa, Salmonella spp., and Staphylococcus aureus; however, serious side effects were described in nerve cells by Tomé, et al. [24].
Observations of clinical signs in animals: Described clinical signs are general responses to the action of macrophages and leukocytes, auxiliaries in the production of IL-1, IL-6, and TNF-α [25]. Most probably, these signs were inhibited by the anti-inflammatory action of the phenolic compounds, such as ellagic and chlorogenic acids, present in SM [26].
The increased scores of pruritus and exudate for the SM 2% GLY24/24 h group in relation to the other treatment groups (p<0.05) may have resulted from the virulence factors of the microorganisms present, for example, leukocidin, deoxyribonuclease, and hyaluronidase from Staphylococcus spp.; adhesins, the mrp operon, importers and exporters of ions, and integrative and conjugative elements called ICE Pm1(PM1 are extremely fine particulates with a diameter of fewer than 1 microns) from Proteus spp. [9]; the transcriptional regulation factor Cra from Citrobacter freundii; the gelatinase (GelE), aggregation factor (Agg), hyaluronidase, and cytolysin (Cyl, β-hemolysin) from Enterococcus spp.; and biofilm formation by Stomatococcus spp. [27-31].
The use of Spondia mombin reduced the number of bacterials and the clinical signs evaluated, proving its effectiveness as a treatment for otitis in dogs. To evaluate the antimicrobial efficacy of an otological solution made from caja leaves for the treatment of bacterial external otitis in dogs. Observations of clinical signs in animals, and Spondia mombim performance in dogs (in vivo testing on dogs). Identification of bacteria and antibiogram and the use of Spondia mombin reduced the number of bacterials. The conclusions are justified on the basis of the study.
This study was funded by the Coordination for the Improvement of Higher Education Personnel (CAPES). We thank the team from the Department of Agricultural and Forestry Sciences at the Federal Rural University of the Semi-Arid Region (UFERSA) for their technical support.
The authors declare no conflicts of interest.
Animal Use Ethics Committee, Universidade Federal Rural do Semi-Árido (UFERSA), number 01/2020, protocol no. 23091.014195/2019-78
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
Citation: Feijo FMC, Silva JS, Alves ND, Rodrigues GSO, Santos CS, Pereira AF, et al. (2022) In Vivo Antibacterial Effectiveness of the Otological Solution Based on Spondias mombin L. in the Treatment of External Otitis in Dogs. J Clin Microbiol Antimicrob 6:127.
Received: 11-Oct-2022, Manuscript No. JCMA-22-19527; Editor assigned: 17-Oct-2022, Pre QC No. JCMA-22-19527 (PQ); Reviewed: 31-Oct-2022, QC No. JCMA-22-19527; Revised: 07-Nov-2022, Manuscript No. JCMA-22-19527 (R); Published: 14-Nov-2022 , DOI: 10.35248/JCMA.22.6.141
Copyright: © 2022 Feijo FMC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.