Chemotherapy: Open Access
Open Access
ISSN: 2167-7700
ISSN: 2167-7700
Research Article - (2023)Volume 11, Issue 2
Background: Chemotherapy-induced neutropenia is the most well-known oncologic emergencies and the most common hematologic toxicity of chemotherapy. A few studies have been carried out to assess the incidence and management practice of chemotherapy-induced neutropenia in Ethiopia.
Objective: The study was conducted to assess incidence, management, and predictors of chemotherapy-induced neutropenia among adult solid cancer patients at the University of Gondar Comprehensive and Specialized Hospital (UOGCSH).
Methods: A hospital-based retrospective follow-up study was conducted among adult solid cancer patients attending between January 1, 2017, to February 30, 2021, at the oncology ward of UOGCSH. A structured data abstraction format was used to collect data from patients' medical charts. Data were analyzed using STATA version 14.2. Bivariate and multivariable logistic regression analysis was used to identify independent predictors of chemotherapy-induced neutropenia and P-value, <0.05 was considered statistically significant. Analysis of variance was used to compare the difference in recovery time of neutropenia between different treatment regimens.
Results: A total of 416 patients were included in the study with a mean age of the patient 50.56 ± 14.4 years. The cumulative incidence of neutropenia was 62.3% (95% CI 57.9-67.1) and 13% of them developed infections. Advanced stage of cancer, poor performance status, patients taking triple treatment modality, lower baseline white blood cell count, elevated lactated dehydrogenase, cisplatin-paclitaxel, doxorubicin-cyclophosphamide, doxorubicincyclophosphamide followed by four cycles of paclitaxel and patients with two or more comorbidities were found to be predictors for chemotherapy-induced neutropenia (P<0.05). The use of filgrastim has significantly reduced the duration of neutropenia recovery time by 33.28 days (P=0.0001) as compared to chemotherapy delay.
Conclusion: The incidence of neutropenia was common in solid cancer and it is multifactorial. Health care professionals should be aware of these risk factors and greater effort is needed to reduce the risk of neutropenia. Filgrastim was the main management for chemotherapy-induced of neutropenia and it was significantly reduced the duration of neutrophil recovery time.
Incidence; Neutropenia; Solid cancer; Treatment; Chemotherapy
ANC: Absolute Neutrophil Count; ANOVA: one-way Analysis of Variance; BMI: Body Mass Index; BSA: Body Surface Area; CIN: Chemotherapy-Induced Neutropenia; CI: Confidence Interval; G-CSF: Granulocyte Colony-Stimulating Factor; LDH: Lactate Dehydrogenase; UOGCSH: University of Gondar Comprehensives and Specialized Hospital; WBC: White Blood Cell
Cancer is one of the leading causes of morbidity and mortality in the world. The world cancer burden was 18.1 million new cases and 9.6 million deaths in 2018 [1]. It is also common in low and middle-income countries, associated with high mortality because patients have been presented in health care institutions after metastasis [2]. According to the national ministry of health in Ethiopia, cancer is estimated to account for about 5.8% of total national death [3]. Chemotherapy and radiotherapy are considered to be the main causes of neutropenia [4,5]. For patients who take chemotherapy treatment, 70% of them develop systemic therapyrelated complications within 4 to 6 weeks of taking the treatment. The previous study indicated that 60,000 persons with cancer were hospitalized with neutropenia and that 88% of participants were considered to have a moderate or major overall impact on life [6,7].
Neutropenia puts the patients at risk for significant morbidity and mortality. The risk of infection correlates with the drop in Absolute Neutrophil Count (ANC) and patients with severe neutropenia are at the greatest risk for life-threatening infections [8].Chemotherapy-Induced Neutropenia (CIN) is the most wellknown oncologic emergencies, the most common hematologic toxicity, and untreated neutropenia is the main cause of lifethreatening infections which leads to a high rate of mortality [9]. CIN is mainly associated with patient-related, disease-related, treatment-related factors [10]. In addition, patients with lower baseline laboratory values such as lower baseline White Blood Cell (WBC), lower baseline platelet count, lower baseline hemoglobin, higher baseline Lactate Dehydrogenase (LDH), and lower baseline albumin are at the highest risk for developing neutropenia [9,11].
CIN is managed by chemotherapy dose modification, dose delay, and/or the initiation of primary prophylaxis with Granulocyte Colony-Stimulating Factor (G-CSF) based on individual risk assessments and the chemotherapy regimen [12]. Currently, the standard treatment for CIN without fever is the use of G-CSF to attenuate white blood cell counts and absolute neutrophil counts. G-CSF has been used frequently to reduce the incidence and duration of CIN [10,12]. In the meantime, cancer is a rapidly evolving disease in Ethiopia due to the increasing smoking, unhealthy diet, physical inactivity, and low early screening practice for different cancers [13]. Even though data on neutropenia were available from the western world; contemporary data from low and middle-income countries including Ethiopia are sparse regarding incidence, management, and predictors of CIN among adult solid cancer patients. In addition, the risk profile of CIN, practice patterns, and healthcare resources in developing countries are different from those of western countries. Therefore, this research will help as one input for the prevention of CIN identifying the most common risk factors.
Study period and area
A hospital-based retrospective follow-up study was conducted among adults’ solid cancer patients attending between January 1, 2017, to February 30, 2021, at oncology ward of the University of Gondar Comprehensive and Specialized Hospital (UOGCSH).
Study design
A hospital-based retrospective follow-up study was employed.
Study population
Patients with a confirmed diagnosis of a malignant solid tumor with age ≥ 18 years, who were on cancer chemotherapy, and who had a normal baseline absolute neutrophil count were included in this study. We excluded patients, who had a history of neutropenia, prior exposure to chemotherapy, and patients with incomplete medical and laboratory records. Because of this group of patients, it was difficult to differentiate the new case of neutropenia from the older existing case.
Sample size determination and sampling technique
The sample size of participants for this study was calculated according to the Cochran formula for sample size calculation for categorical variables. Since there is no previous study conducted in Ethiopia a 50% of incident rate was used in our sample size calculation. The minimum sample size required for this study was 384 based on the standard normal distribution (Z=1.96) with a confidence interval of 95% and a margin of error of 0.05. Finally, after adjusting the 10% non-response rate, 416 participants were included in the final analysis. A simple random sampling technique was used and the proportional allocation was presented (Figure 1).
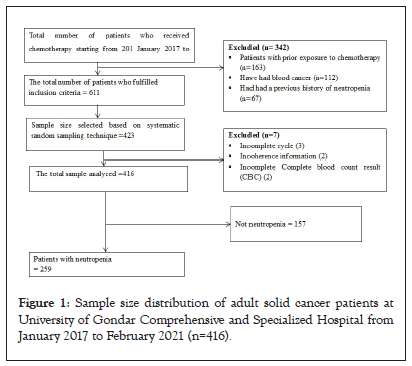
Figure 1: Sample size distribution of adult solid cancer patients at University of Gondar Comprehensive and Specialized Hospital from January 2017 to February 2021 (n=416).
Data collection tool
The data were collected from patient medical charts using a pretested, structured data abstraction form which was prepared by reviewing different kinds of literature. The data abstraction format was composed of detailed information on patient demographics including age, sex, and residency, occupation height, weight, Body Mass Index (BMI), Body Surface Area (BSA), and clinical data including diagnosis/assessments, type of malignancy, chemotherapy agent, number of medications, number of cycle, comorbid medical conditions, amount of absolute neutrophil counts, duration of therapy and other important information.
Data quality assurance
Data collectors were trained by the principal investigator about the data collection method, the appropriate use of the data collection instruments, and the confidentiality of the collected data. They were recruited based on their experience in oncology ward practice that is capable of assessing patients’ past medication experience. The data abstraction tool was pre-tested using 5% of the sample size before actual data collection to assure the internal consistency of the tool so that to meet the objective of the study. Then, the final tool was developed with some modifications after reviewing the results of the pre-test. The scale of reliability coefficient for neutropenia was 80.75% which was good internal consistency. An independent supervisor was closely following the data collection process and the completeness of the data was checked by the principal investigator daily.
Data processing and analysis
The data was entered into a computer database using Epi-data version 4.6 and exported to STATA version 14.2 for outcomes analysis. Categorical variables were summarized as frequencies and proportions. Normal distribution for continuous variables was tested using a histogram. All continuous variables were normally distributed. Simple descriptive statistics such as mean ± SD, frequency, and percentages were determined. Bivariate logistic regression analysis was done to see associations between the dependent and independent variables. Then, independent variables having a P-value of less than 0.25 were included in multivariate logistic regression analysis to identify independent predictors of chemotherapy-induced neutropenia.Those variables having P-value, <0.05 were considered statistically significant. The Hosmer-Lemeshow goodness of fit test for logistic regression was done and the model was well fitted to chemotherapy-induced neutropenia (P=0.96). In addition, a one-way Analysis of Variance (ANOVA) was used to compare neutrophil recovery time between different treatment regimens. A contingency coefficient test was done to see whether categorical variables have multi-collinearity. Variance inflation factor was used to test whether continuous variables had multicollinearity and had an insignificant correlation between variables.
Operational definitions
Absolute neutrophil count: Absolute Neutrophil Count (ANC) (cells/mm3l)=[Total WBC × (Neutrophil % +%band)]/100. Band is not available in the set up and set 0 values [14].
Chemotherapy-induced neutropenia: A reduction of ANC lowers than 1500 cells/mm3 after exposure to chemotherapeutics. Mild, moderate, and severe neutropenia was defined as when ANC is 1000-1500 cells/mm3, 500-1000 cells/mm3, and lower than 500 cells/mm3, respectively [15].
Baseline ANC: ANC value before initiating of first cycle chemotherapy.
Chemotherapy dose delay: a delay of planned chemotherapy for ≥ 7 days [16].
Neutropenia recovery: ANC>1500 cell/mm3 [15].
Performance status: Poor performance status is Eastern Cooperative Oncology Group (ECOG) ( ≥ 2) and good performance status is ECOG (0-I) [17].
Socio-demographic and clinical characteristics of patients
A total of 416 patients were included in the study. The mean age of the patient was 50.56 ± 14.4 years. More than two-thirds of the patients were female 322 (77.4%). Nearest to two-third of the patients were from rural 250 (60.1%). Of the total study participants, 69 (16.6%) were overweight and 5.3% had obesity (Table 1).
| Variable | Category | Frequency | Percent (%) |
|---|---|---|---|
| Gender | Male | 94 | 22.6 |
| Female | 322 | 77.4 | |
| Age | <65 | 374 | 89.9 |
| ≥ 65 | 42 | 10.1 | |
| Residence | Urban | 166 | 39.9 |
| Rural | 250 | 60.1 | |
| Occupation | Housewife | 193 | 46.39 |
| Governmental employer | 108 | 25.96 | |
| Farmer | 73 | 17.55 | |
| Others* | 42 | 10.1 | |
| Marital status | Single | 19 | 4.57 |
| Married | 335 | 80.53 | |
| Divorced | 30 | 7.21 | |
| Widowed | 32 | 7.69 | |
| BSA (m2) | ≤ 1.5 m2 | 142 | 34.13 |
| >1.5 m2 | 274 | 65.87 | |
| BMI (kg/m2) | Underweight (<18.5) | 132 | 31.7 |
| Normal (18.5-24.9) | 193 | 46.4 | |
| Overweight (25-29.9) | 69 | 16.6 | |
| Obesity (≥ 30) | 22 | 5.3 |
Note: BSA: Body Surface Area; BMI: Body Mass Index; * is merchant, student, Person in spiritual schools.
Table 1: Socio-demographic characteristics of adult solid cancer patients at University of Gondar Comprehensive and Specialized Hospital from January 2017 to February 2021 (n=416).
Based on clinical and laboratorial characteristics of study participants, more than half of the patients had good performance status 231 (55.53%) and nearly two-thirds of the patients 268 (64.42%) had an advanced stage of cancer. Regarding treatment modalities, 221 (53.13%) patients were treated by surgery and chemotherapy, and 115 (27.64%) patients were treated by triple treatment modalities. Out of the total study participants, 117 (28.13%) had one comorbidity and 70 (16.82%) had greater than or equal to two comorbidities. Nearly two-thirds 155 (61.3%) of patients had distant metastasis to the liver, lung, bone, and other sites of metastasis (Table 2).
| Variables | Categories | Frequency | Percent (%) |
|---|---|---|---|
| Number of comorbidities | 0 | 229 | 55.05 |
| 1 | 117 | 28.13 | |
| ≥ 2 | 70 | 16.82 | |
| ECOGPS | 0-I (good) | 231 | 55.53 |
| ≥ II (poor) | 185 | 44.47 | |
| Stage of cancer | I-II | 148 | 35.58 |
| III-IV | 268 | 64.42 | |
| Site of distal metastasis | No distal metastasis | 161 | 38.7 |
| Liver and lung | 132 | 31.73 | |
| Bone | 97 | 23.32 | |
| Other sites of metastasis* | 26 | 6.25 | |
| Length of neutropenia resolution time (days) | ≤ 7 | 155 | 59.85 |
| >7 | 104 | 40.15 | |
| Treatment modalities of cancer | Chemotherapy only | 59 | 14.18 |
| Chemotherapy plus surgery | 221 | 53.13 | |
| Chemotherapy plus radiotherapy | 21 | 5.05 | |
| Chemotherapy plus radiotherapy plus surgery | 115 | 27.64 | |
| Treatment intent | Curative | 161 | 38.7 |
| Palliative | 255 | 61.3 | |
| Number of medication per regimen | 1 | 12 | 2.88 |
| 2 | 235 | 56.49 | |
| ≥ 3 | 169 | 40.63 | |
| Number of cycles | 4 | 42 | 10.1 |
| 6 | 274 | 65.87 | |
| 8 | 100 | 24.03 | |
| Baseline laboratory values | Mean ± SD | Reference range | |
| WBC (103 cells/mm3) | 3.5 ± 1.14 | 4-10 | |
| Hgb (g/dl) | 12 ± 2.38 | 12-16 | |
| Lymphocyte (103 cells/mm3) | 3.31 ± 1.12 | 1.2-3.4 | |
| PLT (103 cells/mm3) | 100 ± 19.2 | 144-440 | |
| ANC (103 cells/mm3) | 2.47 ± 0.760. | 2-7.8 | |
| Albumin (g/dl) | 3.5 ± 1.36 | 3.8-4.6 | |
| LDH (U/L) | 596 ± 34.7 | 225-480 | |
| SCr (mg/dl) | 0.79 ± 0.56 | 0.6-1.3 | |
| ALT (U/L) | 24.35 ± 15.77 | ≤ 40 | |
| AST (U/L) | 36 ± 26.42 | ≤ 40 | |
| Sodium (mmol/l) | 136.2 ± 8.36 | 135-145 | |
| Potasium (mmol) | 3.88 ± 0 .98 | 3.5-5.5 | |
| BUN (mg/dl) | 33.7774 ± 16.71 | 15-45 | |
Note: BUN: Blood urea nitrogen; ECOG PS: Eastern Cooperative Oncology Group Performance Status; LDH: Lactate Dehydrogenase; PLT: Platelet Count; WBC: White Blood Cell Count; Hgb: Haemoglobin; SCr: Serum Creatinine; ALT: Alanine Transferase; AST: Aspartate Transferase; ANC: Absolute Neutrophil Count; * is brain, adrenal gland, and peritoneum.
Table 2: Clinical and laboratory characteristics of adult solid cancer patients at University of Gondar Comprehensive and Specialized Hospital from January 2017 to February 2021 (n=416).
Incidence, distribution, and severity of neutropenia among participants at UOGCSH oncology ward
The study includes six common solid cancers in hospitals. Breast cancer was the most commonly diagnosed malignancy 152 (36.54%) followed by colorectal cancer 87 (20.9%). Among patients included in the analysis, A total of 259 (62.3%), 95% CI (57.9-67.1) patients developed neutropenia, and 13% of them developed infections. It was most frequent in breast cancer followed by cervical and ovarian cancer, 77.00%, 70.00%, 68.00%, respectively (Table 3).
| Types of solid tumor | Number of patients n (%) | Neutropenia (%) |
|---|---|---|
| Breast cancer | 152 (36.54) | 117 (77%) |
| Colorectal cancer | 87 (20.91) | 23 (26.4%) |
| Cervical cancer | 67 (16.11) | 47 (70%) |
| Ovarian cancer | 50 (12.02) | 34 (68%) |
| Lung cancer | 34 (8.17) | 21 (61.8) |
| GTN | 26 (6.25) | 17 (65.4%) |
Note: GTN: Gestational Trophoblastic Disease.
Table 3: Distribution of chemotherapy-induced neutropenia among adult solid cancer patients by cancer type at University of Gondar Comprehensive and Specialized Hospital from January 2017 to February 2021 (n=416).
Among patients included in the analysis, a total of 259 patients developed neutropenia, of which 165 (39.66%) had mild neutropenia, 38 (9.13%) had moderate neutropenia, and 56 (13.46%) had severe neutropenia (Table 4).
| Cycles | Neutropenia severity (ANC) n (%) | Total | ||
|---|---|---|---|---|
| Mild (>1000-1500 cells/mm3) | Moderate (500-1000 cells/mm3) | Sever (<500 cells/mm3) | ||
| Post cycle 1 | 9 (2.16) | 38 (9.13) | 15 (3.61) | 62 (14.9) |
| Post cycle 2 | 7 (1.68) | 35 (8.41) | 11 (2.64) | 53 (12.7) |
| Post cycle 3 | 7 (1.68) | 29 (6.98) | 9 (2.16) | 45 (10.82) |
| Post cycle 4 | 6 (1.44) | 22 (5.28) | 7 (1.68) | 35 (8.4) |
| Post cycle 5 | 4 (0.96) | 20 (4.8) | 7 (1.68) | 31 (7.44) |
| Post cycle 6 | 2 (0.48) | 13 (3.13) | 4 (0.96) | 19 (4.57) |
| Post cycle 7 | 2 (0.48) | 6 (1.44) | 2 (0.48) | 10 (2.4) |
| Post cycle 8 | 1 (0.24) | 2 (0.48) | 1 (0.24) | 4 (0.96) |
| Total | 38 (9.13%) | 165 (39.66) | 56 (13.46) | 259 (62.3) |
Note: ANC: Absolute Neutrophil Count.
Table 4: Severity of neutropenia across the cycle among adult solid cancer patients at University of Gondar comprehensive and specialized hospital from January 2017 to February 2021(n=416).
The incidence of chemotherapy-induced neutropenia was commonly occurred at the first cycle of chemotherapy and decreased after the second cycle through the eighth cycle (Figure 2).
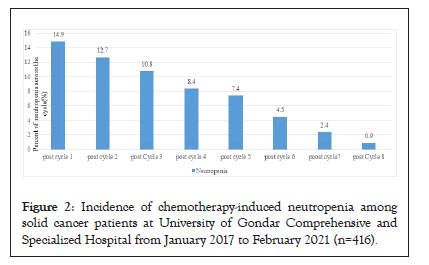
Figure 2: Incidence of chemotherapy-induced neutropenia among solid cancer patients at University of Gondar Comprehensive and Specialized Hospital from January 2017 to February 2021 (n=416).
Types of chemotherapeutic regimens among solid cancer patients at UOGCSH
A total of 416 patients took 416 courses of chemotherapy, 21 types of chemotherapy regimens, and 2708 chemotherapy cycles over all their treatment periods. Over a treatment period, a total of 259 patients suffered from Neutropenia over 479 cycles (17.86%) from the total cycles. The mean cycle of the treatment course was 6.28 ± 1.14. Adriamycin-cyclophosphamide followed 4 cycles of paclitaxel 92 (22.1%), followed by an Adriamycin-cyclophosphamide 86 (20.67%) were commonly prescribed chemotherapy (Table 5).
| Regimens | Total number of patients (%) | Total number of cycles | Neutropenia n (%) | Frequency of neutropenia | NF n (%) | frequency of NF |
|---|---|---|---|---|---|---|
| ACP | 92 (22.1%) | 736 | 66 (15.86) | 126 | 9 (2.2) | 13 |
| Cisplatin and paclitaxel | 86 (20.67%) | 516 | 63 (15.1) | 108 | 13 (3.13) | 15 |
| AC | 59 (14.18%) | 360 | 50 (12.02) | 98 | 6 (1.44) | 7 |
| FOLFOX | 47 (11.3%) | 256 | 9 (2.16) | 21 | 5 (1.2) | 5 |
| CAPOX | 20 (4.8%) | 120 | 8 (1.92) | 16 | 1 (0.24) | 1 |
| Cisplatin and gemcitabine | 18 (4.32%) | 102 | 10 (2.4) | 19 | 2 (0.48) | 2 |
| EMACO | 15 (3.6%) | 120 | 15 (1.2) | 27 | 6 (1.44) | 7 |
| Cisplatin,etoposide and bleomycin | 13 (3.2%) | 78 | 8 (1.92) | 19 | 3 (0.72) | 4 |
| Paclitaxel and carboplatin | 12 (2.88%) | 72 | 10 (2.4) | 14 | 5 (1.2) | 5 |
| FOLFRI | 11 (2.64) | 66 | 3 (0.72) | 5 | 0 (0) | 0 |
| Methotrexate | 11 (2.64%) | 88 | 2 (0.48) | 5 | 0 (0) | 0 |
| Irinotecan and capecitabine | 8 (1.92%) | 48 | 0 (0) | 0 | 0 (0) | 0 |
| Carboplatin and Gemcitabine | 5 (1.2%) | 30 | 4 (0.96) | 5 | 3 (0.72) | 2 |
| Cisplatin and 5FU | 4 (0.96%) | 24 | 2 (0.48) | 2 | 1 (0.24) | 1 |
| Cisplatin and etoposide | 4 (0.96%) | 24 | 2 (0.48) | 2 | 0 (0) | 0 |
| Cisplatin, adriamycin and paclitaxel | 3 (0.72%) | 18 | 0 (0) | 0 | 0 (0) | 0 |
| Cisplatin and adriamycin | 3 (0.72%) | 20 | 3 (0.72) | 7 | 0 (0) | 0 |
| Adriamycin, cyclophosphamide and vincristine | 2 (0.48%) | 12 | 2 (0.48) | 2 | 0 (0) | 0 |
| Cyclophosphamide and cisplatin | 1 (0.24%) | 6 | 1 (0.24) | 3 | 0 (0) | 0 |
| Cisplatin bleomycin and 5FU | 1 (0.24%) | 6 | 0 (0) | 0 | 0 (0) | 0 |
| 5FU and leucovorin | 1 (0.24%) | 6 | 0 (0) | 0 | 0 (0) | 0 |
| Total | 416 (100%) | 2708 | 259 (62.3%) | 479 | 54 (13%) | 62 |
Note: AC: Adriamycin-cyclophosphamide; ACP: Adriamycin-Cyclophosphamide followed by 4 cycles of Paclitaxel; EMACO: Etoposide, Methotrexate, Actinomycin, Cyclophosphamide, and Vincristine; FOLFIRI: Folic acid-Fluorouracil-Irinotecan; FOLFOX: Folic acid- Fluorouracil-Oxaliplatin; CAPOX: Capecitabine Oxaliplatin; 5FU: 5-Fluorouracil.
Table 5: Regimen of chemotherapy administered among adult solid cancer patients UGCSH (n=416).
Determinants of chemotherapy-induced neutropenia among participants
In bivariate logistic regression around 19 variables had a p-value less than 0.25 and candidates for multivariate logistics regression analysis. However, only advanced-stage of cancer, patients with poor performance status, triple treatment modality, WBC<3500 cells/mm3, elevated baseline LDH, cisplatin-paclitaxel regimen, doxorubicin-cyclophosphamide regimen, doxorubicincyclophosphamide followed by paclitaxel, and the presence of two or more comorbidities were significantly associated with an incident of chemotherapy-induced neutropenia.
Accordingly, Patients with advanced-stage of cancer had the risk to develop CIN by odds of 3.3 as compared to patients with stage I and stage II cancer AOR 3.3 (95% CI 1.52-7.12; P=0.0003). Patients with poor performance status had the risk to develop CIN by odds of 3.47 as compared to patients with good performance status AOR 3.47 (95% CI 1.66-7.26; P=0.001). Patients who took triple treatment modality had the risk to develop CIN by odds of 4.74 as compared to patients who took chemotherapy only AOR 4.74 (95% CI 1.39-16.08; P=0.013). Patients with WBC<3500 cells/ mm3 had the risk to develop CIN by odds of 6.27 as compared to patients WBC ≥ 3500 cells/mm3 AOR 6.27 (95% CI 3-13.09; P=0.0001). Patients with elevated baseline LDH had the risk to develop CIN by odds of 5.15 as compared to patients with normal baseline LDH values AOR 5.15 (95% CI 2.42-10.94; P=0.0001). Patients who took the cisplatin-paclitaxel regimen had the risk to develop CIN by odds of 2.89 as compared to patients who did not take the cisplatin-paclitaxel regimen (AOR 2.89, 95% CI1.053- 7.95, P=0.039).
Patients who took the doxorubicin-cyclophosphamide regimen had the risk to develop CIN by odds of 6.08 as compared to patients who did not take the doxorubicin-cyclophosphamide regimen (AOR 6.08, 95% CI 1.76-21.03; P=0.004). Similarly, patients who took doxorubicin-cyclophosphamide followed by paclitaxel regimen had the risk to develop CIN by odds of 3.21 as compared to patients who did not take doxorubicin-cyclophosphamide followed by paclitaxel (AOR 3.21, 95% CI 1.14-9.06; P=0.028). Patients who had two or more comorbidities had the risk to develop CIN by odds of 5.68 as compared to the patient who had no comorbidity (AOR 5.68, 95% CI 1.92-16.8; P=0.002) (Table 6).
| Variables | Categories | Neutropenia (%) | COR (95% CI ) | AOR(95% CI ) | P-value | |
|---|---|---|---|---|---|---|
| No | Yes | |||||
| Gender | Male | 58 (36.9%) | 36 (13.9%) | 1 | 1 | - |
| Female | 99 (63.05%) | 223 (86.1%) | 3.63 (2.24-5.85) | 1.47 (0.65- 3.3) | 0.35 | |
| BSA | ≥ 1.5 | 115 (72.25%) | 159 (61.38%) | 1 | 1 | - |
| <1.5 | 42 (26.75%) | 100 (38.6%) | 1.72 (1.17-2.65) | 1.72 (0.79-3.7) | 0.17 | |
| BMI | Normal | 86 (54.78%) | 107 (41.3%) | 1 | 1 | - |
| Underweight | 25 (15.9%) | 107 (41.3%) | 3.44 (2.04- 5.78) | 1.39 (0.54 -3.56) | 0.49 | |
| Overweight | 39 (24.8%) | 30 (11.6%) | 0.62 (0.35- 1.07) | 1.45 (0.55-3.83) | 0.45 | |
| Obesity | 7 (4.46%) | 15 (5.8%) | 1.72 (0.67- 4.41) | 1.51 (0.39- 5.88) | 0.55 | |
| Stage of cancer | Stage I and II | 106 (67.52%) | 42 (16.21%) | 1 | 1 | - |
| Stage III and IV | 51 (32.5%) | 217 (83.8%) | 10.74 (6.89-17.17) | 3.3 (1.52-7.17) | 0.0003* | |
| Number of comorbidities | 0 | 123 (78.34%) | 106 (40.9%) | 1 | 1 | - |
| 1 | 26 (16.6%) | 91 (35.13%) | 4.06 (2.44- 6.74) | 2.07 (0.93-4.6) | 0.073 | |
| ≥ 2 | 8 (5.1%) | 62 (23.9%) | 8.9 (4.12 - 19.63) | 5.68 (1.92-16.8) | 0.002* | |
| WBC (cells/ul) | ≥ 3500 | 125 (79.6%) | 89 (34.4%) | 1 | 1 | - |
| <3500 | 32 (20.4%) | 170 (65.6%) | 7.46 (4.68-11.81) | 6.27 (3-13.09) | 0.0001* | |
| PLT (cell/ul) | ≥ 100000 | 152 (96.8%) | 227 (87.6%) | 1 | 1 | - |
| <100000 | 5(3.18%) | 32(12.4%) | 4.28 (1.63 -11.24) | 1.45 (0.37-5.55) | 0.59 | |
| Hgb(g/dl) | ≥ 12 | 109 (69.4%) | 123 (47.5%) | 1 | 1 | - |
| <12 | 48 (30.57%) | 136 (52.5%) | 2.51 (1.65-3.81) | 1.7 (0.84-3.45) | 0.139 | |
| Albumin | ≥ 3.5 | 125 (79.62%) | 109 (42.1%) | 1 | 1 | - |
| <3.5 | 32 (20.38%) | 150 (57.92%) | 5.37 (3.39-8.51) | 1.44 (0.68-3.05) | 0.33 | |
| LDH | Normal | 105 (66.87%) | 42 (16.22%) | 1 | 1 | - |
| Elevated | 52 (33.12%) | 217 (83.78%) | 10.43 (6.53 -16.67) | 5.15 (2.42– 10.94) | 0.0001* | |
| AC | No | 148 (94.3%) | 209 (80.7%) | 1 | 1 | - |
| Yes | 9 (5.73%) | 50 (19.3%) | 3.93 (1.88 - 8.25) | 6.08 (1.76 - 21.03) | 0.004* | |
| ACP | No | 131 (83.4%) | 193 (74.52%) | 1 | 1 | - |
| Yes | 26 (16.6%) | 66 (25.5%) | 1.72 (1.04 - 2.86) | 3.21 (1.14-9.06) | 0.028* | |
| FOLFOX | No | 119 (75.8%) | 250 (95.5%) | 1 | 1 | - |
| Yes | 38 (24.2%) | 9 (3.5%) | 0.11 (0.05-0.24) | 0.35 (0.098-1.22) | 0.1 | |
| CAPOX | No | 145 (92.4%) | 251 (96.9%) | 1 | 1 | - |
| Yes | 12 (7.6%) | 8 (3.1%) | 0.39 (0.15-0.96) | 0.39 (0.081-1.85) | 0.235 | |
| FOLFRI | No | 149 (94.9%) | 256 (98.8%) | 1 | 1 | - |
| Yes | 8 (5.1%) | 3 (1.2%) | 0.22 (0.057-0.84) | 0.19 (0.028- 1.28) | 0.089 | |
| Methotrexate | No | 148 (94.3%) | 257 (99.2%) | 1 | 1 | - |
| Yes | 9 (5.7%) | 2 (0.77%) | 0.13 (0.027- 0 .6) | 0.55 (0.042-7.23) | 0.65 | |
| ECOG | 0-I | 128 ( 81.5%) | 103 (39.8%) | 1 | 1 | - |
| ≥ 2 II | 29 ( 18.5%) | 156 (60.2%) | 6.68 (4.16- 10.73) | 3.47 (1.66-7.26) | 0.001* | |
| Paclitaxe- Cisplatin | No | 134 (85.4%) | 196 (75.7%) | 1 | 1 | - |
| Yes | 23 (14.6% ) | 63 (24.3%) | 1.87 (1.1-3.2) | 2.89 (1.053-7.95) | 0.039* | |
| Treatment modalities of cancer | Chemotherapy only | 31 (19.75%) | 28 (10.8%) | 1 | 1 | - |
| Chemotherapy plus surgery | 94 (59.87%) | 127 (49%) | 1.49 (0.84 -2.66) | 2.05 (0.75-5.56) | 0.156 | |
| Chemotherapy plus radiotherapy | 8 (5.1%) | 13 (5.01) | 1.79 (0.649 - 4.98) | 1.8 (0.39-8.23) | 0.45 | |
| Chemotherapyradiotherapyplus surgery | 24 (15.3%) | 91 (35.1%) | 4.19 (2.12- 8.29) | 4.74 (1.39-16.08) | 0.013* | |
Note: AOR: Adjusted Odds Ratio; COR: Crude Odds Ratio; CI: Confidence Interval; LDH: Lactate Dehydrogenase; ECOG: Eastern Cooperative Oncology Group; FOLFIRI: Folinic Acid Fluorouracil Irinotecan, FOLFOX: Folinic Acid- Fluorouracil Oxaliplatin; CAPOX: Capecitabine Oxaliplatin; AC: Adriamycin Cyclophosphamide, ACP: Adriamycin Cyclophosphamide followed by 4 cycles of Paclitaxel; WBC: White Blood Cell Count; Hgb: Haemoglobin; PLT: Platelet Count. Significance (P<0.05).
Table 6: Predictors chemotherapy-induced neutropenia among adult solid cancer patients at University of Gondar Comprehensive and Specialized Hospital from January 2017 to February 2021 (n=416).
Management of neutropenia
Chemotherapy-induced neutropenia was managed by chemotherapy delay (did not start next planned chemotherapy for ≥ 7 days), filgrastim, and filgrastim with anti-bacterial. The mean duration of filgrastim treatment was 3.2 ± 0.73 days. Comparison using ANOVA test showed that use of filgrastim was significantly reduced the duration of neutropenia recovery time by 33.28 days (P=0.0001) as compared to chemotherapy delay. In contrast, the addition of ciprofloxacin to filgrastim had no significant difference in recovery time of neutropenia as compared to the filgrastim-only regimen (P=0.86) (Table 7).
| Type of interventions | Frequency n (%) | Mean time neutropenia recovery(days) |
|---|---|---|
| Filgrastim | 126 (48.7) | 11.32 |
| Chemotherapy delay | 101 (39) | 44.6 |
| Filgrastim+ciprofloxacin | 32 (12.3) | 11.88 |
| One way ANOVA test of neutrophil recovery time among neutropenia patients | ||
| Filgrastim only vs. chemotherapy delay | -33.28 (P=0.0001) | |
| Ciprofloxacin+filgrastim versus chemotherapy delay | -32.72 (P=0.0001) | |
| Filgrastim only versus filgrastim+ciprofloxacin | -0.56 (P=0.86) | |
Table 7: Management of chemotherapy-induced neutropenia among adult solid cancer patients at UOGCSH from January 2017 to February 2021 (n=416).
The objective of this study was to determine the incidence, management, and predictors of chemotherapy-induced neutropenia particularly in patients with breast cancer, colorectal cancer, lung cancer, ovarian cancer, cervical cancer, and gestational trophoblastic disease undergoing chemotherapy. Patients with solid malignancy receive several and frequent chemotherapies as per patient in clinical practice. Consequently, chemotherapy-induced neutropenia and its complication is a known source of major stress for physicians and patients [18].
In this study, the cumulative incidence of chemotherapy-induced neutropenia was 62.3% (95% CI 57.9-67.1) and 13% of them were developed infections in patient with general poor conditions. The incident of CIN was in line with a study done in Brazil (63.3%) [19]. However, this result was higher than previous studies done in Japan (50.5%), and Portuguese (8%) [17,20]. This might be due to our study participants being blacks who had lower neutrophil count and leucocyte count relative to white people [21]. In addition, primary prophylaxis was not common practice in our setup, all patients in our study had new cancer cases and on first chemotherapy exposure which had relatively more sensitive to chemotherapy toxicity relative to recurrent cases [17]. It was also higher than a study conducted in Nigeria (31%) [22]. This might be due to the use of primary prophylactic G-CSF based on risk stratification is not common in our setup. On the other hand, the incidence in our study was lower as compared to a previous study done in Nepal (80.3%) [14]. This variation might be due to differences in the use of chemotherapy regimens, Nepal used highrisk chemotherapy for breast cancer as compared to intermediaterisk and low-risk chemotherapy regimens in our study.
Our study showed that the incidence of CIN was higher in breast cancer than in other types of cancers. This finding was in line with other studies conducted previously in the world [23,24]. This might be due to patients taking high-risk chemotherapy regimens doxorubicin, cyclophosphamide, and taxane-based regimen, which have a high risk of bone marrow suppression [25,26]. The incidence of the neutropenia episode was higher at the first (14.9%) and the second cycles (12.7%) of therapies and decreased in the subsequent cycles. The finding was in line with other previous studies conducted in the United States, Europe, and Denmark [27- 29]. This might be because patients had lower tolerability levels at the onset of chemotherapy [25]. The mean duration of neutropenia was 38.4 days, which was comparable with a study conducted in Kenya (34.6 days) [30]. In contrast, it was longer than the previous study in Belgium (4 days) [22]. This might be due to a lack of frequent complete blood count monitoring as per standards which is important for the detection of neutropenia events immediately.
This study showed that elevated baseline LDH was significantly associated with CIN. The finding was in line with the previous reports conducted in the USA and Thailand [31-33]. Elevation of LDH in cancer patients might be a result of tumor burden and increased glycolytic activity of tumor cells which is an indicator of metastasis that increased myelosuppression [34]. Lower baseline WBC count was also a significant factor for the incidence of CIN. This result was in line with previous studies in Europe, and China [35,36]. WBC (neutrophils) is the first defense against infection and inflammation. The lower baseline WBC might be a result of the direct consequence of cancer [34].
Poor performance status was significantly associated with CIN. This factor was in line with the previous studies in Japan, and Nigeria [17,36]. This might be due to decreasing physiological age can increase easily fragility and myelosuppression. The advanced stage of cancer was also a risk factor for CIN. This finding was in line with a previous study in Japan, France, and Korea [17,35,37]. When cancer became advanced osteoplastic and osteolytic effects of cancer can lead to bone marrow aplasia which leads to chemotherapy-induced myelosuppression particularly, neutropenia [22].
Doxorubicin-cyclophosphamide, doxorubicin-cyclophosphamide followed by paclitaxel regimen, cisplatin-paclitaxel regimen, and combination treatment modality of chemotherapy, radiotherapy, and surgery was significantly associated with the incidence of neutropenia. The result was consistent with the studies in Denmark, Belgium, and Korea [37-39]. This effect was also in line with the recommendation of the National Comprehensive Cancer Network (NCCN) guideline [40]. These chemotherapies have high bone marrow suppression effects as compared to other drugs [41,42]. Patients with two or more comorbidities were significantly associated with CIN. The finding was supported by a study conducted in Korea [43]. This might be due to several underlying pathologic mechanisms, including bone marrow suppression, impaired neutrophil, and other immune cell function, disturbance of barrier functions, increased availability of pathogenic microbes [44,45].
Regarding the management of neutropenia, nearly two-thirds of the patients were managed by filgrastim, and filgrastim plus ciprofloxacin. These regimens have significantly reduced the duration of neutropenia by 33.3 days and 32.73 days respectively as compared to chemotherapy delay. However, there was no significant difference in recovery time of neutropenia between filgrastim, and filgrastim plus ciprofloxacin. The result was consistent with a previous retrospective observational study conducted in Malaysia [46]. It was also similar to a study conducted in Netherland [47]. Therefore, clinicians should be given attention to the addition of ciprofloxacin to filgrastim which might increase the treatment cost and antibiotic resistance without significantly reducing neutropenia recovery time.
This study found that nearly two-thirds of patients taking chemotherapy developed new cases of neutropenia in solid tumors. Neutropenia occurs most frequently during the first and second cycles of chemotherapy than subsequent cycles. Advanced stage, poor performance status, patients taking triple treatment modality, lower baseline white blood cell count, elevated LDH, cisplatin-paclitaxel, doxorubicin-cyclophosphamide, doxorubicincyclophosphamide followed by four cycles of paclitaxel and patients with two or more comorbidities were risk factors for chemotherapyinduced neutropenia. The majority of neutropenia patients were managed by using filgrastim, and filgrastim plus antibiotics. However; there was no significant difference in recovery time between filgrastim, and filgrastim plus antibiotics.
Risk factors of chemotherapy-induced neutropenia are multidimensional, health care professionals should be aware of these risk factors. Greater effort is needed to reduce the risk of neutropenia including screening of risk factors to start early prophylaxis and treatment. In addition, more researches or large surveys; prospective follow-up study in a different clinical setting requires assessing the overall situation.
Despite we have tried to invest our best efforts through the study, it may not be out of any limitation. The study was conducted in a single center; the findings may not be generalized to reflect the health care setting in Ethiopia. We used a retrospective follow-up study, the necessary data such as chemotherapy dose reduction, previous antibiotics use, and duration of therapy, might have been missed. In addition, quality of life including nutritional status might be affecting the incidence, of chemotherapy-induced neutropenia; however, this study is unable to address these factors. Despite these limitations, the present study provides new insights into the incidence, management, and predictors of chemotherapyinduced neutropenia among adult solid cancer patients; could serve as a source of direction as it identified areas for intervention.
Ethical clearance with ethical approval code SOPs/133/2021 was obtained from the ethical clearance committee of the department of clinical pharmacy, school of pharmacy, University of Gondar. Permission to access the medical records of patients was then being obtained from UOGCSH clinical directorate. According to Federal regulation and recent FDA guidance informed consent was waived by institutional review board of UOGCSH as the study was conducted retrospectively and involves no more than minimal risk. This study was conducted following the Declaration of Helsinki. Confidentiality of the patients was ensured in such a way that the data was only be used for the study purpose. To ensure privacy and confidentiality, information collected was not directly linked to the respective participants; names of patients were not used. Codes were used as an identifier.
Not applicable
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
The authors declared that they have no conflict of interests.
This research was funded by the University of Gondar, School of Pharmacy, College of Medicine, and Health Science. The University has no role in designing, conducting, and reporting the study.
Samuel Agegnew conceptualized study, study design and data analysis, Sumeya Tadesse took part in study design and analysis, Dessie Abebaw took part in interpretation of data, Samuel Berihun Dagnew took part in analysis, Ephrem Mebratu Dagnew take part in conceptualized the study design, Eyayaw Ashete Belachew interpreted the result and revised manuscript, Bekalu Kebede drafted the initial manuscript. All authors read and approved the final manuscript.
We thank the participants of the study, the University of Gondar, and hospital record room staff for their time and facilitation of the data collection process.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Wondm SA, Tadesse S, Abebaw D, Dagnew SB, Dagnew EM, Belachew EA, et al. (2023) Incidence, Management and Predictors of Chemotherapy-Induced Neutropenia among Adult Solid Cancer Patients at the University of Gondar Comprehensive and Specialized Hospital: Retrospective Follow up Study. Chemo Open Access. 11:182.
Received: 21-Feb-2023, Manuscript No. CMT-23-21870; Editor assigned: 24-Feb-2023, Pre QC No. CMT-23-21870 (PQ); Reviewed: 10-Mar-2023, QC No. CMT-23-21870; Revised: 17-Mar-2023, Manuscript No. CMT-23-21870 (R); Published: 24-Mar-2023 , DOI: 10.35248/2167-7700.23.11.182
Copyright: © 2023 Wondm SA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.