Pancreatic Disorders & Therapy
Open Access
ISSN: 2165-7092
+44 1478 350008
ISSN: 2165-7092
+44 1478 350008
Research - (2022)Volume 12, Issue 4
Background: Single cell suspension culture is the prime requirement for cell culture studies as that in clinical medicine. For disaggregating cells, use of enzyme: trypsin has been the first choice by researchers. Commercially, trypsin is obtained from bovine source which adds to the cost of the experiment. Present investigation highlights on utilization of fish waste derived trypsin as a cell dissociating agent anticipated to reduce environmental footprint.
Methods: The current study thus aimed at studying the efficacy of fish waste derived trypsin over commercial counterpart as a cell-dissociating agent. The enzyme isolated from Catla catla visceral waste was purified by Gel filtration chromatography, and characterized by peptide analysis BLAST analysis. The efficacy in terms of cell viability was checked over cancerous cell lines procured from NCCS, Pune, India.
Results: The crude enzyme extract isolated from Catla catla visceral waste exhibited 145.22 mg.ml-1 protein content which was enhanced to 152.93 mg.ml-1 after purification. Upon performing BLAST analysis of the peptides, the enzyme was found to be trypsin. The enzyme (0.1% and 1% concentration) retained 90% cell viability at 10 sec when compared with bovine source.
Conclusion: The fish visceral waste was considered as a novel source of trypsin enzyme which demonstrated an endurable cell dissociating ability.
Fish visceral waste; Cell lines; Trypsinisation; Homogenisation; Precipitation
Clinical research involves culturing of cells and their analysis for which cells need to be in suspension. However, many others are adherent to the substratum which hinders their accessibility towards analysis. To overcome this difficulty, suitable cell detachment methods viz. physical and chemical or a combination of both, are used. Trypsinisation is an enzymatic process breaks the integrin- ligand bonds responsible for cell attachment to the substratum [1] thus exhibits same efficacy as mechanical method. Trypsinisation is a time-dependent process and commercially obtained from bovine source (Figure 1). However, the aversive response of trypsin over cells has been reported by Huang HL [2]. They do so by cleaving the cell membrane proteins. Cells have also been reported to undergo altered gene expression levels along with proteome [3]. Therefore, trypsinization should be performed with caution, by optimizing both the duration of trypsinization and the concentration of trypsin.
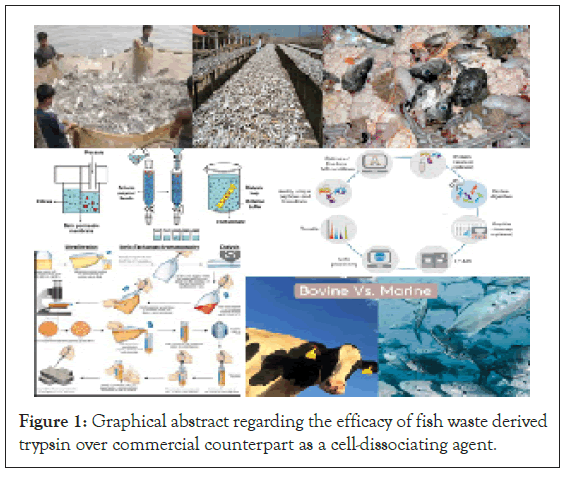
Figure 1: Graphical abstract regarding the efficacy of fish waste derived trypsin over commercial counterpart as a cell-dissociating agent.
Transformation of fish visceral waste into useful products is considered as an advantageous promising alternative for environmental and economic sustainability. Fish visceral waste has been the source of amino acids, metabolites and many bioactive compounds [4]. Poikilotherms have a wide diversity of feeding and habitat preferences as a result, their enzymes are also diversified. Among the enzymes in industrial demand, proteases rank first and have exhibited fastest growth in past decade [5]. Characterization of the enzyme trypsin isolated from fish visceral waste has been reported by several scientists but their application as a cell-dissociating agent have not been studied. Utilization of marine fishery derived waste products in clinical medicine have been emphasized by Takahashi [6], but till date, application of freshwater fish derived waste has not been underlined. The authors have suggested a unique application of such waste as a source of trypsin which could be used as a cell dissociating agent. Trypsin was characterized by measuring amidase activity and efficacy in terms of cell viability. For comparison, trypsinisation was also achieved using commercial trypsin (Himedia). The study was conducted over cancerous cell line: KB cell line.
Collection and maintenance of cell lines
To study trypsinisation in cells, KB cell lines: Cancerous in nature (HeLa derivative) were chosen for the study (Figure 2) and obtained from national centre for cell sciences, pune. These do not have any strict growth requirements and are not influenced by adverse growth conditions, thus were selected. The cell lines were cultured and timely passaged using RPMI-1640 media. To maintain gaseous environment, CO2 incubator (New Brunswick, an eppendorf company) was used.
Figure 2: Microphotograph of KB cell line (40X).
Procurement of trypsin source
The indigenous source of trypsin was fish visceral waste. The waste was collected in an ice-box from local fish market and transferred to Molecular Biology Laboratory, Department of Biotechnology, Barkatullah University, Bhopal. 100 g viscera was washed with 0.8% saline to remove blood and debris, labelled and stored at -20Ë?C for further use. The freshwater carps chosen for the study- Catla catla (C) possessed following characteristics:
Compressed body, broad head, wide mouth, thin upper lip covered by skin of snout, thick lower lip and circular opening of gills (Figure 3). According to Talwar PK and Jhingaran AG [7], these are the features of Catla catla. The fin formula, as described by Rahman AA is-D. 2/15-16; P1.18-20; P2. 9; A. 3/5.[8]
Figure 3: pancreatic-disorders-viscera-12-4-238-g003
Preparation of crude extract
As given in the method of Simpson BK and Haard NF [9], a 10% homogenate of Catla catla was prepared in extraction buffer (1 mM Tris-HCl and 1 mM CaCl2; pH 8). With the aim of obtaining a homogenate, the suspension was agitated at 200 rpm at 4Ë?C for 30 min (ORBITEK Orbital Shaker, Scigenics- Biotech) followed by centrifugation (Eppendorf Centrifuge, 5415R) at 11,400 g at 4Ë?C for 30 min. The supernatant, referred to as Crude Enzyme Extract (CEE) was stored in deep freezer (Blue star) at -20Ë?C and protein concentration was determined by Lowry’s method [10].
Clarification of extract
Precipitation or fractionation is usually done to clarify the crude extract to recover the proteins. This can be achieved by either using salts or by solvents. Present investigation employed both solvent (cold acetone, and TCA: Acetone) and salt (NH4 (SO4)2), but cold acetone precipitation was found 24.48% more effective over salt precipitation [11]. Cold acetone has been found as a lipid removal agent but deteriorates protein quality [12].
Purification and characterisation of trypsin
Trypsin was purified using DEAE-cellulose column (0.5 × 5.5 cm) and extraction buffer as eluent. The fractions were collected and their absorbance was measured at 280 nm. The fraction with highest absorption peak was selected for further characterization (Figure 4). The amidase activity in purified trypsin was determined according to the method of Erlanger BF [13] using N-α-benzoyl-DL-arginine P-nitroanilide (BAPNA) as substrate. The enzyme was digested and the resulting peptides were subjected to Orbitrap High Resolution Liquid Chromatography Mass Spectrometry (O-HRLCMS). The conventional charge-based separation techniques provide a bird’s eye view of the molecular status whereas peptide analysis provides a rather comprehensive and specific profile of the protein. The sequences were thus aligned with those of other fish trypsin using BLAST tool to establish similarity (Table 1).
| S. No. | Retention time (min) | Position | Sequence | Organism | BLAST results | |||
|---|---|---|---|---|---|---|---|---|
| Max score | Query coverage | Identity | Accession number | |||||
| 1 | 13.93 | May-14 | IEVRLGEHNI | Labeo rohita | 35.8 | 100% | 100% | RXN10210.1 |
| 2 | 14.01 | 117-124 | TMFCAGYL | Labeo rohita | 31.2 | 100% | 100% | KAI2667855.1 |
| 3 | 14.07 | 120-131 | CAGYLEGGKDSC | Trematomus bernacchii | 41.4 | 100% | 100% | XP_033998327.1 |
| 4 | 24.93 | 77-88 | VTGWGNTMSPTA | Carrasius auratus | 58.7 | 100% | 100% | XP026104836.1 |
Table 1: Characteristics of peaks obtained and their percentage identity.
Figure 4: Total Ion Current Chromatogram (TIC) of digest of fraction 1.
Efficacy of trypsin
As explained by Freshney RI [14], the efficacy of trypsin in animal cell culture was determined. Three sets of enzyme concentration were set viz., 0.01%, 0.1% and 1%, each at three time intervals of 10 sec, 15 sec and 20 sec (Figures 5-7). To check cell viability, cells were removed from the solution and resuspended in 10 ml of growth media. A 10 μl of inoculum (20,000 cells after 48 hrs) was incubated with equal volume of trypan blue solution at room temperature for 1 min. The number of cells and cell viability percentage was calculated using hemocytometer as,
Cells per ml=viable cells × D × 5000
Where,
D=dilution factor
Viability (%) was calculated as,
Viability=(number of live cells/number of dead cells) × 100
All the values are presented as mean ± standard deviations carried out in triplicates. The data was analyzed using Analysis of Variance (ANOVA) and values significant above confidence level 95% (p<0.05) were accepted.
Figure 5: Microphotograph at 0.01% concentration of (a) Control for 10 sec, (b) Control for 15 sec, (c) Control for 20 sec, (d) Sample for 10 sec, (e) Sample for 15 sec, (f) Sample for 20 sec (40X)
Figure 6: Microphotograph at 0.1% concentration of (a) Control for 10 sec, (b) Control for 15 sec, (c) Control for 20 sec, (d) Sample for 10 sec, (e) Sample for 15 sec, (f) Sample for 20 sec (40X)
Figure 7: Microphotograph at 1% concentration of (a) Control for 10 sec, (b) Control for 15 sec, (c) Control for 20 sec, (d) Sample for 10 sec, (e) Sample for 15 sec, (f) Sample for 20 sec (40X)
The KB cell lines were successfully established and regularly passaged. The fish from which viscera was obtained, was identified as Catla catla by FishBase, an online database. In the crude extract protein content was found as 145.22 mg ml-1 which was enhanced to 152.93 mg.ml-1 after purification. Lamas [15] and Geethanjali and Subash [16] have independently reported cold acetone as an effective precipitating agent. The absorption peaks of the fractions were obtained (Figure 8) and that exhibiting highest absorbance was chosen for further analyses. Upon analysing the TIC, further interrogation by MASCOT search engine and BLAST analysis proved that the enzyme extracted was similar to the enzyme trypsin [17]. Using same technique, He [18] have also identified the peptides generated by digestion of vitellogenin protein isolated from three different fishes-Pimephales promelas, Micropterus salmoides, and Fundulus heteroclitus.
Figure 8: Absorption peaks of all the five collected fractions of partially
purified enzyme. Note:  fraction 1;
fraction 1;  fraction 2;
fraction 2;  fraction 3;
fraction 3; 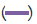 fraction 4;
fraction 4; 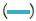 fraction 5.
fraction 5.
The effectiveness of Catla catla trypsin was determined in terms of cell viability at three different concentrations (0.01%, 0.1% and 1%) and three different time intervals (10 sec, 15 sec and 20 sec) (Figure 9). The cell viability was observed highest at 10 sec time interval for both control and sample. These results were in accordance with that reported by Vijayakumar [19] who observed similar viability but at 0.25% trypsin concentration. The reason may be use of commercial trypsin in place of fish derived crude form of trypsin.
Figure 9: Cell viability at different concentrations. Note:  Control;
Control; 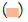 Sample
Sample
The extracted enzyme from Catla catla visceral waste was found similar to commercial bovine trypsin. The affectivity of the enzyme in trypsinisation was comparable with that of commercial counterpart. On the basis of present studies, it can be revealed this trypsin can replace the commercially available enzyme in terms of cost, efficacy and waste management. A shift from linear to circular economy is the need of hour, which is based upon redesigning along with the 3R’s (Reusing, Recycling and Reducing). However, the unavoidable side effects of trypsin over the proteome of the cell have raised concern in this regard. Thus, the waste can be minimized in an effective manner.
Prof. Ragini Gothalwal conceived the idea and Dr. Charu Batav collected the data; Prof. Ragini Gothalwal and Dr. Charu Batav analyzed the data; and Dr. Charu Batav wrote the paper.
The authors like to express their gratitude to the Department of Biotechnology, Barkatullah University, Bhopal, for providing laboratory facility.
[PubMed]
Citation: Batav C, Gothalwal R (2022) Indigenous Trypsin: An Underpinning Approach in Clinical Medicine. Pancreat Disord Ther. 12:238.
Received: 16-Aug-2022, Manuscript No. PDT-22-19300(PQ); Editor assigned: 19-Aug-2022, Pre QC No. PDT-22-19300; Reviewed: 01-Sep-2022, QC No. PDT-22-19300; Revised: 07-Sep-2022, Manuscript No. PDT-22-19300 (R); Published: 15-Sep-2022 , DOI: 10.35248/2165-7092.22.12.238
Copyright: © 2022 Batav C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.