Medicinal & Aromatic Plants
Open Access
ISSN: 2167-0412
ISSN: 2167-0412
Research Article - (2021)Volume 10, Issue 2
Breast cancer is the most common type of cancer in women. Despite current treatments, new treatments should be investigated. The antioxidant and apoptotic activities of plant metabolites have attracted increasing interest in the molecular pharmacology. Here, we investigated the anti-proliferative and apoptotic potential of Myrtus communis L. extracts, used in traditional medicine. To monitor cancer-selective activities, the extract and its most prominent components were assayed in parallel with human MCF7 breast cancer and normal MCF10A mammary epithelial cells. Indeed, we observed a potential therapeutic window of concentrations with significantly higher toxicity for the tumor cells. GC-MS confirmed the presence of α-pinene, 1,8-cineole, linalool as major components. Analyzing these pure terpenoids revealed exclusive toxicity of 1,8-cineole for MCF7 cells, whereas no toxicity was seen for MCF10A cells. In contrast, both α-pinene and linalool did not exhibit tumor selectivity but were toxic for the tumor and normal cells. These data suggest that the anti-proliferative activity of the oil may be mediated by 1,8-cineole with the other components counteracting this potential therapeutic activity, which apperantely acted preferentially on epithelial cells. To gain mechanistic insight, we tested by RT-qPCR whether the essential oil affected apoptosis mediators. Indeed, BCL2 expression was dramatically down-regulated in MCF7 while it was up regulated in MCF10A. Minor differences were observed for BAX, while APAF1 expression was up-regulated in MCF7 and down-regulated in MCF10A. Our data suggest that in M. communis extracts, 1,8-cineole mediates tumor-selective apoptosis by deregulating BCL2 and APAF1.
Myrtus communis L.; 1,8-cineole; Breast cancer; Cytotoxicity; Apoptosis
Breast cancer is one of the most common malignancies in women. In 2020, there were 2.26 million new cases of breast cancer and 684,996 deaths from breast cancer [1]. Familial cases for earlyonset breast cancer revealed the predisposition due to mutations in the BRCA1 and BRCA2 genes [2]. Apart from a diversity of additional gene mutations, identified general risk factors included post-menopausal age, weight, alcohol intake [3] and hormone replacement therapy [4]. A number of features have been described as hallmarks of cancer which can be acquired during the multistep development of human tumors. These features are a) sustained proliferative signaling, b) growth suppressor evasion, c) cell death resistance, d) replicative immortality, e) induction of angiogenesis, and f) activation of invasion and metastasis, as well as immune escape and altered metabolism [5].
Standard therapy for breast cancer depends on the classification and includes classical surgery, chemo or radiation therapy. More recently, hormonal therapy for ER+ (Estrogen Receptor-Positive) cancers, antibody, and immune checkpoint therapies have been added and gene targeting therapies are being developed [6]. Given the genotoxicity of conventional radiation and chemotherapies, research in bioactive natural compounds and nature-inspired synthetic structures has identified novel types of cancer therapeutics [7]. Indeed, natural or natural compound-derived products-like penicillin or taxanes-correspond to the most important drugs in modern medicine. The predominant role of natural products in cancer are obvious from the fact that approximately 74% of anticancer compounds are of natural origin [8].
M. communis is a well-known medicinal plant used worldwide in traditional medicine (Figure 1A). This species belonging to the Myrtaceae family, which includes 100 genera and 3,000 species, is perennial, in the form of a bush, green in summer and winter, usually short and 1-3 meters tall [9]. M. communis is traditionally used for the treatment of diverse disease conditions [10]. The essential oil of M. communis has a rich content of terpenes (monoterpene hydrocarbons and sesquiterpene hydrocarbons), terpenoids (oxygenated monoterpenes and oxygenated sesquiterpenes), and phenylpropanoids [11]. Several of the monoterpenes have been reported to have antitumor activity, exhibiting the ability not only to prevent cancer formation or progression but also to regress existing malignant tumors [12]. Analysis of M. communis extracts done by GC-MS (Gas chromatography-Mass spectrometry) identified linalool (Figure 1B), 1,8-cineole (Figure 1C), α-pinene (Figure 1D) and as major compounds.
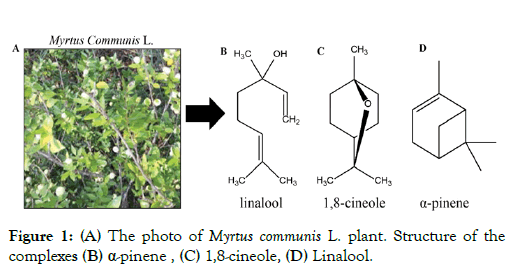
Figure 1: (A) The photo of Myrtus communis L. plant. Structure of the complexes (B) α-pinene, (C) 1,8-cineole, (D) Linalool.
Previously anticancer studies using M. communis oil have been reported [13,14] but in none of these cases of cancer cell selectivity has been investigated. This is however necessary to reveal a potential therapeutic effect. Therefore, in this study we investigated the cytotoxic and apoptotic effects of M. communis essential oil and its constituents in parallel in tumor MCF7 and normal MCF10A mammalian epithelial cells. We report here that one of these terpenes exerts exclusive tumor selectivity most likely due to the divergent induction of apoptosis mediators in normal and tumor cells.
The flow sheet of the experimental analysis using MCF7 breast cancer and MCF10A normal breast epithelial cells as well as BJ human fibroblast is presented in Figure 2.
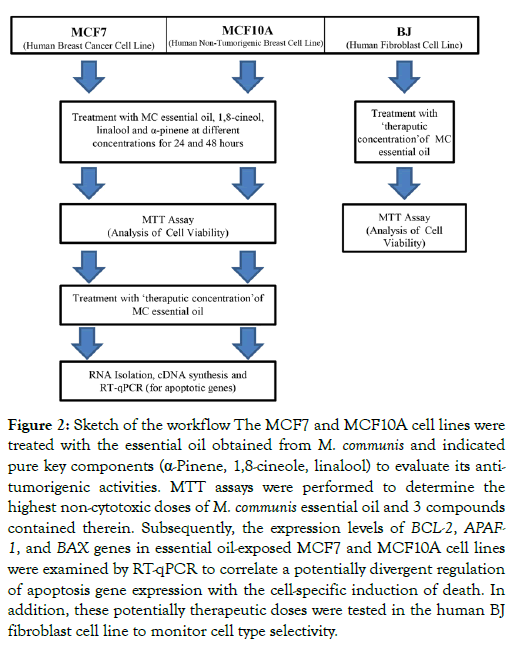
Figure 2: Sketch of the workflow The MCF7 and MCF10A cell lines were treated with the essential oil obtained from M. communis and indicated pure key components (α-Pinene, 1,8-cineole, linalool) to evaluate its antitumorigenic activities. MTT assays were performed to determine the highest non-cytotoxic doses of M. communis essential oil and 3 compounds contained therein. Subsequently, the expression levels of BCL-2, APAF- 1, and BAX genes in essential oil-exposed MCF7 and MCF10A cell lines were examined by RT-qPCR to correlate a potentially divergent regulation of apoptosis gene expression with the cell-specific induction of death. In addition, these potentially therapeutic doses were tested in the human BJ fibroblast cell line to monitor cell type selectivity.
Essential oil preparation and pure compounds
Essential oils of M. communis are obtained from the Telkaliş trial areas of Hatay Mustafa Kemal University, Faculty of Agriculture, Department of Medical and Aromatic Plants. α-pinene, linalool and 1,8-cineole were purchased from Sigma. The essential oil was prepared exactly as described [15]. The oil was dried over anhydrous sodium sulfate and then stored in dark color glass bottles and keep in the refrigerator (about 4°C) until use for analysis.
GC-MS Analysis
Analysis of the components of essential oils was carried out by using a Thermo Scientific ISQ Single Quadrupole Gas Chromatograph equipped with MS (Mass Spectra) detection (5% phenyl polysilphenylenesiloxane, 0.25 mm inner diameter × 60 m lengths, 0.25 μm film thickness). The carrier gas was helium (99.9%) at a flow rate of 1 mL/min; ionization energy was 70 eV. Mass range was m/z 1.2-1200 amu. Data acquisition was in scan mode. MS transfer line temperature was 250°C, MS ionization source temperature was 220°C, the injection port temperature was 220°C. The column temperature was 50°C at the beginning and it was increased to 220°C at 3°C/min heat increase rate [15]. The structure of each compound was identified by comparison with their mass spectrum (Wiley 9 library). The data was handled using the Xcalibur software program (Version 2.1.0 SP1, Thermo Fisher Scientific, Milan, Italy). Thirty-seven compounds were identified in the extract of M. communis. Representative GC-MS chromatography is shown in Supplementary Figure 1 and the corresponding compounds with their retention times, match factor, reverse match factor, and concentrations (%) are shown in Supplementary Table 1.
Preparation of M. communis essential oil doses
The M. communis essential oil and its components were prepared with a serum-free medium containing DMSO (Dimethyl Sulfoxide, SIGMA). Stock solutions of M. communis essential oil and its components were prepared with DMSO at 3 mg/mL and 1.2 mg/mL. Serial dilutions were done by a serum-free medium with maximal final DMSO concentrations of 0.1%.
Cell culture
The human breast epithelial cancer MCF7 cell line, the human non-tumorigenic breast epithelial MCF10A cell line, and the human fibroblast BJ cell line cells were provided by the cell culture service of the Institute of Genetics and Molecular and Cellular Biology (IGBMC), France. A PHCbi biosafety cabinet and a Memmert incubator were used in the cell culture. The MCF7 cells were grown in Dulbecco's Modified Eagle's Medium with 4.5% glucose (DMEM) supplemented with 10% fetal bovine serum (FBS), 0.6 μg/mL insulin, and 40 μg/mL gentamycin. The MCF10A cells were grown in DMEM/HAM F12 (1:1) supplemented with 5% horse serum, 20 ng/mL human epidermal growth factor, 100 ng/mL cholera toxin, 0.01 mg/mL insülin, 500 ng/mL hydrocortisone, 40 μg/mL gentamycin. The BJ ATCC cells were grown in Minimum Essential Medium (MEM) with Earle's Balanced Salts supplemented with 10% FBS, 1 mM sodium pyruvate, 40 μg/mL gentamycin, and non-essential amino acids. All cells were incubated in 75 cm2 flasks in 5% CO2 and 37°C incubator under sterile conditions.
MTT assay
MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide) assays were carried out as previously described [16]. 96- well plates were inoculated with 1 × 104 cells per well and incubated for 24 h. Cells were exposed in triplicates to serial dilutions of the extracts and the pure compounds (1,8-cineole, α-pinene, linalool) at concentrations from 3 mg/mL to 18.75 μg/mL as specified in the figures. 10 μL of 5 mg/mL MTT in PBS was added to each well and incubated at 37°C for 4 h. After removal of the MTT, formazan crystals were dissolved using 100 μL DMSO and incubated 37°C for 0.5 h. Cell viability was monitored by measuring absorbance at 590-690 nanometers against the reference wavelengths using a Biochrom EZ Read 400 spectrophotometer. IC50 was defined as the concentration that inhibited 50% of cell growth (Supplementary Figure 2).
Quantitative real-time PCR analysis
Exponentially growing of MCF7 and MCF10A cell were treated with M. communis essential oil at 600 μg/mL for a 24 h. RNA was isolated using Trizol as described [17]. RNA quality and quantity determined by using a Thermo Scientific Varioskan LUX microplate reader. RNAs were stored at -80°C. cDNA synthesis was done using High Capacity cDNA Reverse Transcription Kit (Applied Biosystems by Thermo Fisher Scientific). RT-qPCR reactions were done with a Roche Light Cycler 480 device. Expression levels of BAX, BCL2 and APAF1 were quantitated relative to GAPDH gene expression levels. BAX, BCL2 and APAF1 oligonucleotides were designed using the primer3 program [18] (Supplementary Table 2). MFEPrimer- 3 program [19] was used to evaluate PCR primer specificity, dimers and hairpin structure. Oligo Analyzer program was used to evaluate primer quality, homo and heterodimer formation.
Statistical analysis
All cell experiments were performed as biological triplicates. To determine IC50, GraphPad Prism Version 8.0 was used and SPSS 23 were used to calculate and compare the mean values. The Kolmogorov-Smirnov analysis was used to verify if results followed a normal distribution. Inter-group measurements were compared using one-way ANOVA for variables with normal distribution and Shapiro-Wilk variance analysis for variables without normal distribution. Each test group was compared with the control group and statistical significance was p<0.05. Analysis of the expression data performed on the QIAGEN Rotor-Gene Q tool was done with RT2 profiler PCR Array Data Analysis Version 3.5 and GAPDH was used as a 'housekeeping gene' for normalization.
All experiments were performed by using a single master extract of essential oil which was kept at 4°C in the dark. It has been previously shown that essential oils can have biological activities which effect proliferation and/or apoptosis [13]. However, it has never been evaluated, whether these effects were due to general toxicity or whether they exhibited cancer-cell selectivity. Here we have used a comparative approach with normal MCF10A and tumor MCF7 cells to see whether we can identify cancer cell-specific effects.
Dose-response curves established by the MTT test revealed that M. communis essential oil decreased the viability of MCF7 breast cancer cells more pronounced than that of MCF10A normal cells at concentrations above 375 μg/mL (Figure 3). Corresponding IC50 (the half-maximal inhibitory concentration) values are provided in Supplementary Figure 2. This data suggested that the essential oil contained one or more components that are toxic to both cell lines. However, the preferential killing of MCF7 cells below a concentration of 375 μg/mL suggested that the oil contained ingredients which may exert tumor cell selectivity.
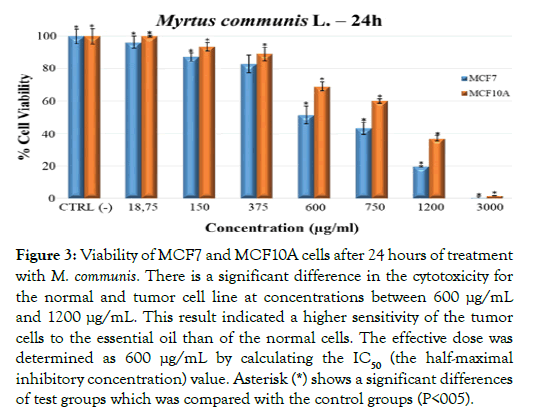
Figure 3: Viability of MCF7 and MCF10A cells after 24 hours of treatment with M. communis. There is a significant difference in the cytotoxicity for the normal and tumor cell line at concentrations between 600 μg/mL and 1200 μg/mL. This result indicated a higher sensitivity of the tumor cells to the essential oil than of the normal cells. The effective dose was determined as 600 μg/mL by calculating the IC50 (the half-maximal inhibitory concentration) value. Asterisk (*) shows a significant differences of test groups which was compared with the control groups (P<005).
Previously, bioactive metabolites of M. communis have been described [20,21]. Using GC-MS, we positively identified 37 components (Supplementary Table 1). The main components of the essential oil of the M. communis were α-pinene (31.82%), 1,8-cineole (21.33%), myrtenyl acetate (14.01%), linalool (8.20%). To assess the potential contribution three of these compounds to the differential effect of the essential oil on MCF7 tumor and MCF10A normal cells, we established dose-response curves with the pure compounds. MTT assays revealed that both α-pinene and linalool were toxic for the two cell lines (Figures 4A and 4B). The normal cells were even more affected than the tumor cells. This relative toxicity was not only reversed for 1,8-cineole but this particular compound displayed virtually no toxicity for normal MCF10A cells up to very high concentrations (Figure 4C). In a stark contrast, MCF7 viability was already affected at 375 μg/mL and decreased progressively towards higher concentrations. These data suggest that the essential oil contains at least two bioactive metabolites (linalool and α-pinene) which are toxic for both normal and tumor cells. In contrast, one ingredient of the essential oil, namely 1,8-cineole, had no toxic effect at a concentration where it was able to kill up to 40% of the tumor cells. The effect of the essential oil is likely a composite of the effects of the tumor-selective and non-selective compounds. Note in this respect that not all of the components of the essential oil have been tested with pure compounds. It is thus possible that other ingredients may contribute to the final effects of the essential oil. Several bioactive metabolites have been previously described to exert cytotoxic effects in tumor cell lines, including linalool [22-24] α-pinene [25-27] and 1,8-cineole [28,29]. However, as for the essential oil, tumor-cell selective effects of these components have not been demonstrated. To study if the effect of the M. communis oil was different in different cell types, we compared the cell viabilities of breast epithelial MCF7 and MCF10A cells with that of BJ fibroblasts at a concentration of 600 μg/mL (Figure 5).
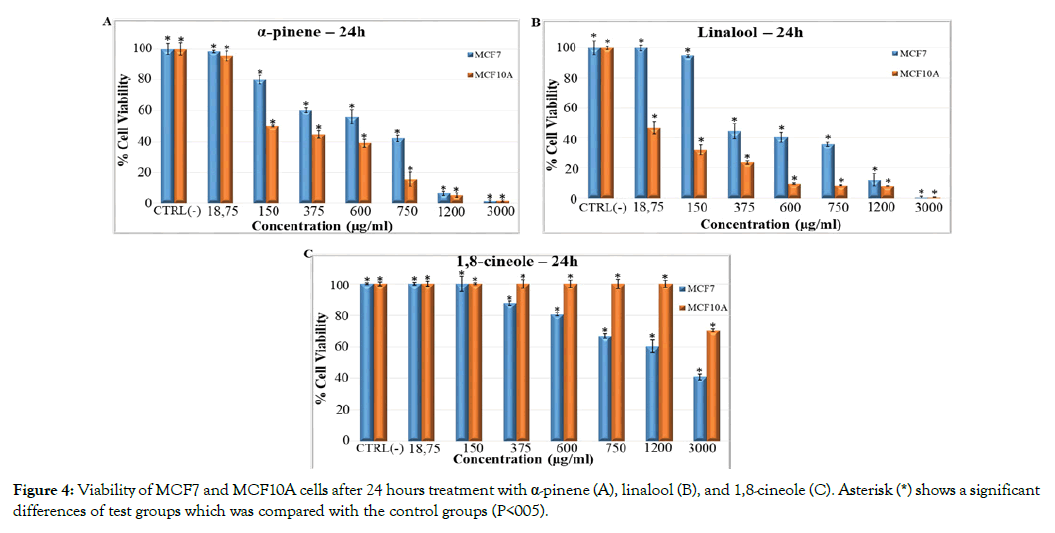
Figure 4: Viability of MCF7 and MCF10A cells after 24 hours treatment with α-pinene (A), linalool (B), and 1,8-cineole (C). Asterisk (*) shows a significant differences of test groups which was compared with the control groups (P<005).
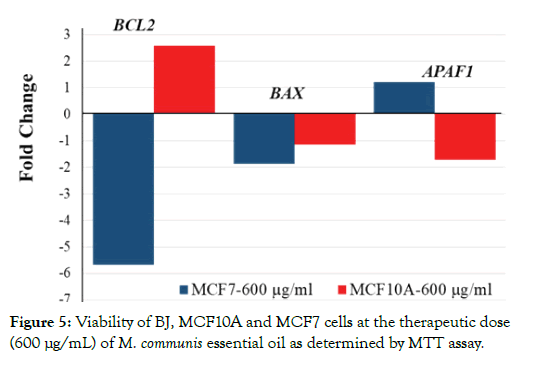
Figure 5: Viability of BJ, MCF10A and MCF7 cells at the therapeutic dose (600 μg/mL) of M. communis essential oil as determined by MTT assay.
Notably, there was a very small effect (about 10%) on the viability of BJ cells. The fact that cell viability was affected in a cell-specific manner, suggested to us that the effects of M. communis oil may be due to cell-specific death signaling. One of the most prominent cell death signaling pathways is apoptosis [30]. As apoptosis is governed by the balance between pro and anti-apoptotic factors, we tested three of those factors by quantitative RT-PCR. We aimed to test the hypothesis that the essential oil could affect their expression levels. Indeed, as shown in Figure 6, the expression of both the anti-apoptotic BCL2 and pro-apoptotic APAF1 were divergently regulated in MCF7 and MCF10A cells. Even more importantly BCL2 expression was decreased in MCF7 cells while APAF1 was increased. These data indicate that the essential oil increased apoptosis in the tumorigenic MCF7 cells. In contrast, the opposite was observed in MCF10A cells suggesting that increased expression of BCL2 and decreased APAF1 expression rather stabilized the survival of normal breast epithelial MCF10A cells. The above results suggest that one of the bioactive metabolites (1,8-cineole) of M. communis essential oil is non-toxic for normal cells while it induces apoptosis in MCF7 tumor cells. The tumor-selective effect of 1,8-cineole is likely to be partly disguised by the general toxicity of linalool and α-pinene, thus accounting for the limited tumor cell selectivity of the essential oil. Our study is unique in that plant extracts and several of its components were studied for tumorselective cytotoxicity in parallel in normal and tumor cells. Several other plant extracts and components have been shown to affect the expression of apoptosis-mediators [31-34] but tumor cell selectivity has not been demonstrated.
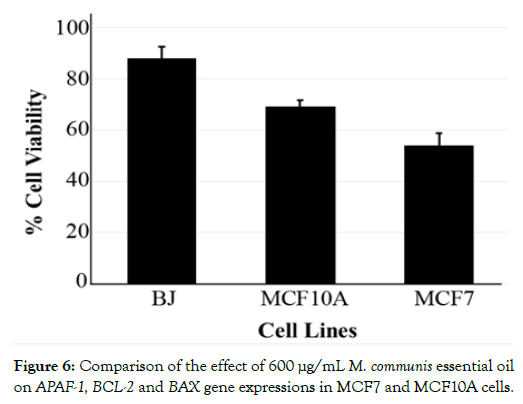
Figure 6: Comparison of the effect of 600 μg/mL M. communis essential oil on APAF-1, BCL-2 and BAX gene expressions in MCF7 and MCF10A cells.
Our approach may be useful as a general concept for identifying plant extract-derived bioactive components for cancer therapy. While MCF10A and MCF7 cells are not isogenic, future studies may take advantage of the possibility of using primary human cell lines and their engineered tumorigenic variants. Moreover, our comparative analyses with BJ fibroblasts suggest incorporating different cell types in such a screening protocol, to monitor cell type-specific and tumor cell-specific activities of extracts and individual bioactive compounds.
The authors declare that they have no competing financial interests.
Kasikci Y was initially supported by a fellowship of the EU Erasmus Program. This project was supported by Cukurova University Research Fund as project number TYL-2018-11209. Studies in the Gronemeyer H laboratory by funded from the Plan Cancer, AVIESAN_ITMO Cancer, the Ligue National Centre le Cancer (HG; Equipe Labellisée); and the Institut National du Cancer (INCa).
Kasikci Y and Karacay S performed all cell culture studies. Kasikci Y did all experimental studies involving MTT analysis, RNA isolation and cDNA analysis. Pazarbasi A and Yilmaz MB supervised the cell culture studies. Kaya DA prepared M. communis essential oil and did the GC-MS analysis. Kumar A performed RT-PCR experiments. Gronemeyer H and Pazarbasi A conceived and designed the study and Kasikci Y wrote the manuscript with the help of HG.
Citation: Kasikci Y, Pazarbasi A, Karacay S, Kaya DA, Duran GG, Kumar A, et al. (2021) Induction of Tumor-Selective Cytotoxicity by Myrtus communis L. Extract and its Bioactive Metabolite 1, 8-cineole. Med Aromat Plants (Los Angeles) 10: 371.
Received: 02-Feb-2021 Accepted: 20-Feb-2021 Published: 27-Feb-2021 , DOI: 10.35248/2167-0412.21.10.371
Copyright: © 2021 Kasikci Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.