
Journal of Clinical Toxicology
Open Access
ISSN: 2161-0495

ISSN: 2161-0495
Research Article - (2021)Volume 11, Issue 3
Arsenite (NaAsO2) is a potent toxin that significantly contributes to human pathogenesis. Chronic exposure to arsenite results in various diseases. The physiologically important biological target(s) of arsenite exposure is largely unknown. Here we found that transient sodium arsenite treatment (1) blocks nigericin or Rotenone induced IL- 1β secretion; (2) inhibits mitochondrial respiration with complex I-linked substrate; (3) induces Heme oxygenase-1 (HO-1) in myocardial tissue, (4) attenuates the myocardial ischemia-reperfusion injury in an in vivo model of rats. The causal relationship among these activities needs further investigation.
Arsenite; Myocardial ischemia-reperfusion; NLRP3 inflammasome; Mitochondria; Mitochondrial complex; Citric acid cycle; Heme oxygenase-1
HO-1: Heme Oxygenase-1; I/R: Ischemia/Reperfusion; LV: Left Ventricular; eIF2α: Eukaryotic Translation Initiation Factor 2 Alpha; As2O3: Arsenic Trioxide; APL: Acute Promyelocytic Leukemia; LAD: The Left Anterior Descending; TTC: 2,3,5-Triphenyltetrazolium Chloride; EF: Ejection Fraction; FS: Fractional Shortening; PMA: Phorbol-12-Myristate-13-Acetate; BMDM: Bone-Marrow-Derived Macrophages; dTGN: Dispersed Trans-Golgi Network; INF/AAR: Infarct Size To Area-At-Risk.
CMyocardial Ischemia-Reperfusion (I/R) injury is an important clinical issue. Great efforts have been devoted to alleviate I/R injury which can greatly improve the patient's prognosis [1]. The first few minutes of reperfusion are critical, because what happens then initiates long-term tissue damage and dysfunction [2]. Studies showed an important role for mitochondrial activity early in IR injury. The first damaging event upon reperfusion is a burst of Reactive Oxygen Species (ROS) production from mitochondria [3-5]. Mitochondrial ROS not only causes acute damage but also drives successive pathology including activation of the innate immune system and initiate the local sterile inflammatory response following reperfusion [2,5].
The mitochondrial ROS production is linked to the citric acid cycle and the state of the respiratory complex. Ischemia could alter the abundance of many mitochondrial metabolites that can potentially act as electron stores and CoQ reductants upon reperfusion. Succinate was found to accumulate significantly during ischemia and its level controlled the ROS production in heart during reperfusion [6]. The Complex I is proposed to be the major source of mitochondrial superoxide during Reperfusion [5]. Complex I is the entry point for electrons from NADH into the mitochondrial respiratory chain and is well established as a major source of mitochondrial superoxide in vitro. Selective pharmacological inhibition of complex I protects heart from reperfusion injury and ROS production [7-9].
Following reperfusion, NLRP3 inflammasome mediates the sterile inflammatory response triggered by tissue damage and plays a role in the pathogenesis of myocardial ischemia-reperfusion injury [10-12]. Blocking inflammasome activation by genetic method markedly diminishes infarct development and myocardial fibrosis and dysfunction [10,11]. In a screening for chemicals that influence inflammasome activation, one hit that drew our attention was the compound sodium arsenite. Arsenic is a metalloid that generates various biological effects both on mitochondria and cytoplasm [13- 18]. Acute arsenite toxicity is considered as oxidation of cysteine residues in target proteins that directly alters their conformation or activity. Arsenite was reported to inhibit the enzymatic activity of Pyruvate Dehydrogenase (PDH) in mitochondria. Arsenic intensively affects the ROS-metabolizing enzymes called antioxidant enzymes, such as SOD, CAT, Glutathione Peroxidase (GPx), GST, and Glutathione Reductase (GR). Generally short-term exposure to low arsenic concentrations results in an increase in the activity of these enzymes, whereas chronic exposure usually results in their reduction [13]. Cells exposed to arsenite start a cytosolic stress response including phosphorylation of eukaryotic translation initiation factor 2 alpha (eIF2α) and elevation of downstream genes such as CHOP, GADD34 and heat shock proteins which are important against stressful insults [15-20]. Sodium arsenite also induces Heme Oxygenase-1 (HO-1) which protects against ischemia and reperfusion injury in mice [21-23]. Considering the pros and cons of arsenite on cells, we wonder its effect on myocardial ischemia-reperfusion injury. The in vivo myocardial ischemiareperfusion model in rats was made to explore the effect of arsenite on myocardial tissue during the first 24 hour.
Cell culture and inflammasome stimulation
Human THP-1 cells were grown in RPMI 1640 medium, supplemented with 10% FBS. THP-1 cell were differentiated for 3 hour with 100 nM Phorbol-12-Myristate-13-Acetate (PMA). Bone marrow was flushed from mouse femurs and cultured in bone marrow media (RPMI 1640 containing 20% heat-inactivated fetal bovine serum, 30% L929 cell media, 2 mM L-glutamine) for 7 days at 37°C in 4 100-mm polystyrene dishes to obtain mature, differentiated macrophages.
For inducing IL-1β, 106 macrophages were plated in 12-well plate overnight and the medium was changed to opti-MEM in the following morning, and then the cells were primed with ultrapure LPS (100 ng/ml) for 3 hour. After that, arsenite were added into the culture for 5 minutes, and then the cells were stimulated with ATP (5 mM, pH adjusted to 7.5) or Nigericin (10 μM) for 1 hour, or rotenone (10 μM) for 3 hour.
Immunostaining
U2OS or HeLa cells were plated on coverslips for 1 day and transfected with pcDNA6-flag-hNLRP3 vector for 36 hours. After that, the cells were treated with nigericin (10 μM) for 30 minutes and stained with antibody.
For immunostaining, cells were fixed with 4% paraformaldehyde for 15 minutes and permeabilized with 0.1% Triton X-100 in PBS, before incubation with anti-FLAG M2 antibody followed by Cy3 secondary antibodies. Nuclei were stained with DAPI.
Isolation of mitochondria
Deprive a 150 g to 200 g male Sprague-Dawley rat of food overnight. Liver was taken out immediately after animal was sacrificed and washed with ice-cold LHM (0.2 M mannitol, 50 mM sucrose, 10 mM KCl, 100 mM HEPES, 1 mM EDTA). Then the liver was minced finely and washed with LHM again. The minced tissue was homogenized with Dounce homogenizer and centrifuged at 1000 g at 4°C for 5 minutes to remove nuclei and tissue debris. The supernatant was transferred and centrifuged at 4000 g at 4°C for 10 minutes. The pellet suspended with LHM was homogenized 3 to 4 times gently again and centrifuged at 4000 g at 4°C for 10 minutes. The pellet was washed with LHM again. The resulting pellet was re-suspended in respiration medium.
Respiration measurements
Respiration measurements in isolated mitochondria were conducted at 25°C in a Clark type oxygen electrode (INESA Scientific Instrument, REX, JPB-607A portable Dissolved Oxygen Meters). The respiration medium consisted of 0.225 M mannitol, 55 mM sucrose, 10 mM KH2PO4, 12 mM KCl, 5 mM MgCl2, 10 mM Tris-HCl pH 7.4. Mitochondria were transferred to a electrode chamber placed on a magnetic stirrer and the volume was adjusted to 2 ml. Mitochondria were treated with arsenite or not 4 minute before oxygen consumption was recorded. The effects of arsenite on mitochondrial respiration were determined in the presence of excess ADP (1 mM), using substrate combinations targeting either Complex I (5 mM glutamate plus 2 mM malate) or Complex II (5 mM succinate plus 1.25 μM rotenone) of the respiratory chain.
in vivo myocardial I/R
SD rats (250-300 g) were intraperitoneally anesthetized with sodium Pentobarbital (40 mg/kg), orally intubated with Teflon tubes (1.2/1.6 mm), and connected to a rodent ventilator. A left thoracotomy was then performed. The chest muscle was set aside, and the third and fourth ribs were break off. The area around left auricle and pulmonary arterial cone was exposed. The Left Anterior Descending (LAD) coronary artery was ligated using 6-0 silk suture around a fine tube with a firm knot. Rats were subjected to 40 minutes of LAD ischemia followed by 24 hour of reperfusion. The infarct area was determined by 2, 3, 5-Triphenyltetrazolium Chloride (TTC) staining. All animal studies were conducted in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals. All animal experiments performed in this study adhered to the protocols approved by the Institutional Animal Care and Use Committee of Zhengzhou University.
Infarct area assessment
After 1 day of reperfusion, rats were anesthetized, Evans blue solution (3%, 1 ml) were directly injected into the right ventricle, 1 minute later, the heart was quickly excised, immediately frozen, and sliced. Sections were then incubated in a 1% TTC solution which was freshly prepared, and the slices must be covered with a glass sheet during staining. After staining, sections were digitally photographed and the infarct area was determined by computerized planimetry.
Echocardiography
After 24 hour of reperfusion, echocardiography was performed using a VEVO 2100 high-resolution in vivo imaging system (FUJIFILM Visual Sonics, Toronto, Ontario, Canada). Under anesthesia, the chest of the rat was shaved, and two-dimensional echocardiography was performed. Cardiac function was evaluated by M-mode. Left Ventricular (LV) end-diastolic diameter and LV end-systolic diameter were measured on the parasternal LV longaxis or short-axis view. Left ventricular Ejection Fraction (EF) and Fractional Shortening (FS) were calculated with computerized algorithms. All measurements represented the mean of five consecutive cardiac cycles.
Arterial pressure measurements
After 24 hours of reperfusion, before TTC staining method was performed, mice were anesthetized. The femoral artery was dissected and was cannulated using a catheter pre-filled with heparinized normal saline (0.5 IU/ml). After cannulation, the other end of the catheter was connected to the transducer. The arterial systolic pressure and diastolic pressure was monitored by a biological Signal Acquisition and Analysis System (BL-420F, TECHMAN, Chengdu, China).
Western blotting
The antibodies for phospho-eIF2α Ser51 were purchased from elabscience (E-AB-20864). The antibodies for eIF2α (sc-133132), GADD34 (sc-373815), GADD153 (sc-7351) and Heme Oxygenase 1 (sc-136960) were from Santa Cruz biotechnology.
Mice intraperitoneally injected with arsenite (4.5 ug/g) for various periods were sacrificed and organs were excised quickly. Protein was extracted from frozen tissue by grinding using a pre-chilled mortar. The RIPA lysis buffer (50 mM Hepes pH 7.5, 150 mM NaCl, 2 mM EDTA, 2 mM EGTA, 1% TritonX-100, 50 mM NaF, 5 mM Sodium Pyrophosphate, 50 mM Sodium glycerophosphate, 1 mM NaVO3, 1 mM DTT, 1 mM PMSF, 10 g/ml Leupeptin, 10 g/ml Aprotinin) was used for protein preparation. Following SDSPAGE, nitrocellulose membranes were blocked with 3% skim milk, and immunoblotting was performed as described elsewhere.
RT-PCR
Total RNA was extracted from hearts, brain and liver tissue of rats intraperitoneally injected with PBS or arsenite (4.5 ug/g) for 7 hours. Semi-quantitive PCR were performed to detect the mRNA expression. The following primers were used: HO-1 (5'-GACCGTGGCAGTGGGAATT-3' a n d 5 ' - T G G T C AG T C A AC A T G G AC G C - 3 ' ) , C H O P ( 5 ' - AC T C T TGAC C C T G C AT C C C T- 3 ' a n d 5 ' -T C TC AT T C T C C TG C TC C T T C T C - 3 ' ) , X B P 1 ( 5 ' -T TAGTGTC TA A AGCCACCCAC C - 3 ' and 5'-GCCAGGCTGAACGATAACTG-3'), GAPDH (5'-TGGAAAGCTGTGGCGTGAT-3' and 5'-GGGTGGTCCAGGGTTTCTT.
Statistical analysis
Data are presented as mean ± standard deviation. Comparisons between groups were performed by Student’s two-tailed unpaired t-test. Statistical significance was set at P<0.05.
Arsenite blocks nigericin or Rotenone induced IL-1β secretion
In a screening for chemicals that acted on inflammasome activation, we found that sodium arsenite strongly inhibited IL-1β secretion. To determine the effects of arsenite on NLRP3 inflammasome activation, Bone-Marrow-Derived Macrophages (BMDMs) or THP-1 cells were treated with arsenite before LPS priming or before agonist challenge. We observed that arsenite blocked IL-1β secretion at both situations. It has been reported that arsenite can inhibit protein synthesis [13] and NF-kB activation [24], we then examined whether arsenite had an impact on LPS-induced priming for inflammasome activation. As shown in Figure 1, Arsenite inhibited LPS-induced pro-IL-1b, pro-caspase-1 and NLRP3 expression.
It’s recently reported that recruitment of NLRP3 to dispersed trans-Golgi network (dTGN) is an early and common cellular event that leads to NLRP3 aggregation and activation [25,26]. Different NLRP3 stimuli lead to disassembly of the TGN. To explore whether arsenite has any effect on the recruitment of NLRP3 to dTGN, U2OS and HeLa cells transfected with flag tagged NLRP3 were treated first with arsenite and then with nigericin. Fluorescence microscopy experiments showed that flag_NLRP3 aggregated around nucleus in U2OS or formed small dots in HeLa cells upon nigericin treatment, whereas arsenite decreased the NLRP3 aggregation (Figure 2).
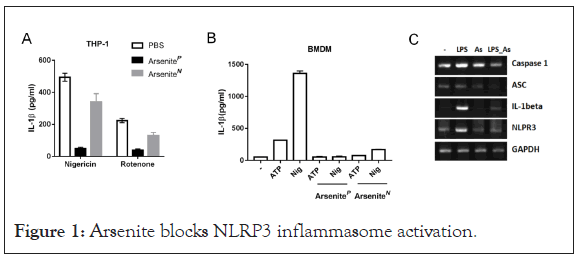
Figure 1: Arsenite blocks NLRP3 inflammasome activation.
(A) THP-1 cells were primed with LPS (100 ng/ml) for 3 hour and were stimulated with nigericin (5 μM) for 1 hour or Rotenone (10 μM) for 3 hours. Sodium arsenite (40 μM) was added into medium together with LPS (ArseniteP) or with Nigericin (ArseniteN). Supernatants were analyzed by ELISA for IL-1β. Experiments were repeated three times.
(B) LPS-primed Bone-Marrow-Derived Macrophages (BMDMs) were stimulated for 1 hour with ATP (5 mM) or Nigericin (10 μM), Sodium arsenite (10 μM) was added into medium together with LPS (ArseniteP) or with Nigericin (ArseniteN). Supernatants were analyzed by ELISA for IL-1β. Experiments were repeated three times.
(C) BMDMs treated with LPS and arsenite alone or collectively for 4 hours were analyzed by RT-PCR “As” Sodium arsenite.
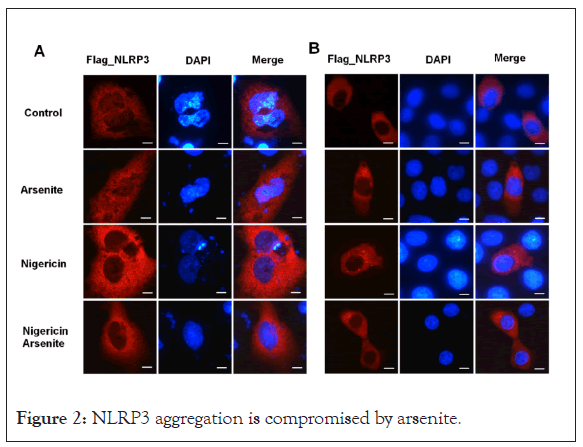
Figure 2: NLRP3 aggregation is compromised by arsenite.
(A) U2OS and (B) HeLa cells transfected with Flag_NLRP3 vector were treated with nigericin and sodium arsenite alone or collectively for 30 minutes, cells were fixed and stained with FLAG antibody. Scale bars: 10 μM
Inhibition of mitochondrial respiration by high dose arsenite
AArsenite was reported to have an effect on mitochondria. We explored its role on respiration rate. Arsenite at 200 μM produce a strong inhibition of oxygen consumption in isolated rat liver mitochondria with complex I-linked substrate (Figures 3B and 3C), but did not have a substantial effect on complex II-linked respiration using succinate as the substrate (Figure 3A). Arsenite at 20 μM did not show a significant effect on complex I (Figure 3C).
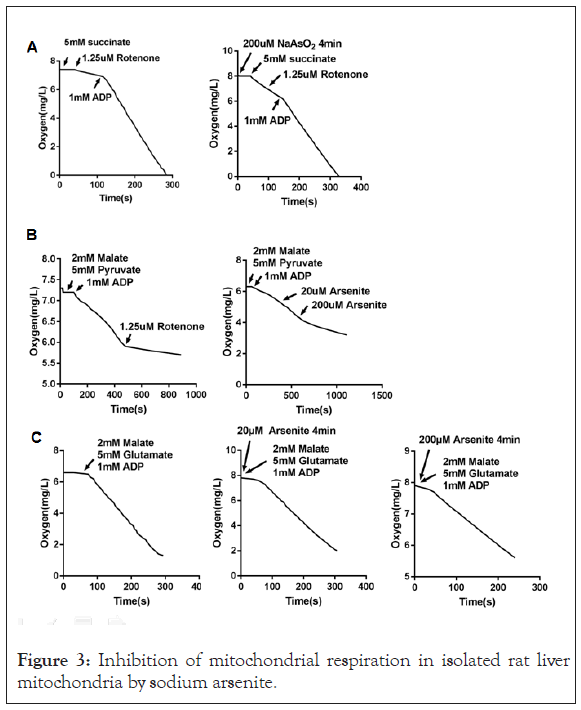
Figure 3: Inhibition of mitochondrial respiration in isolated rat liver mitochondria by sodium arsenite.
(A) Mitochondria were treated with sodium arsenite for 4 minutes or not, and then oxygen consumption rates were measured using substrate (5 mM succinate plus 1.25 μM rotenone) targeting respiratory complex II.
(B) Oxygen consumption rates were measured using substrate (2 mM malate plus 5 mM Pyruvate) targeting respiratory complex I. Rotenone or NaAsO2 were added into the reaction buffer as the arrows showed.
(C) Mitochondria were treated with sodium arsenite for 4 minutes or not, and then oxygen consumption rates were measured using substrate (2 mM malate plus 5 mM glutamate) targeting respiratory complex I.
Arsenite attenuates myocardial ischemia-reperfusion injury
It was reported arsenic trioxide induced apoptosis in cancer cells and here we found that arsenite inhibits NLRP3 inflammasome activation and mitochondrial respiration which were involved in myocardial ischemia/reperfusion injury, thus we wondered what effect arsenite has on myocardial I/R injury. The in vivo model of myocardial ischemia-reperfusion was used to answer this question. Rats were subjected to 40 minutes of LV ischemia and 24 hour reperfusion. Arsenite was administered by intraperitoneal injection 30 minutes before ligation of the Left Anterior Descending (LAD) coronary artery at dose of 4.5 mg/kg. Evaluation of infarct size revealed a significant cyto-protection effect of arsenite as assessed by 2, 3, 5-Triphenyltetrazolium Chloride (TTC) staining. Surprisingly, rats receiving 4.5 mg/kg arsenite displayed a lower ratio of infarct size to area-at-risk (INF/AAR), as shown in Figures 4A and 4B (Supplementary Figures 1 and 2 ).
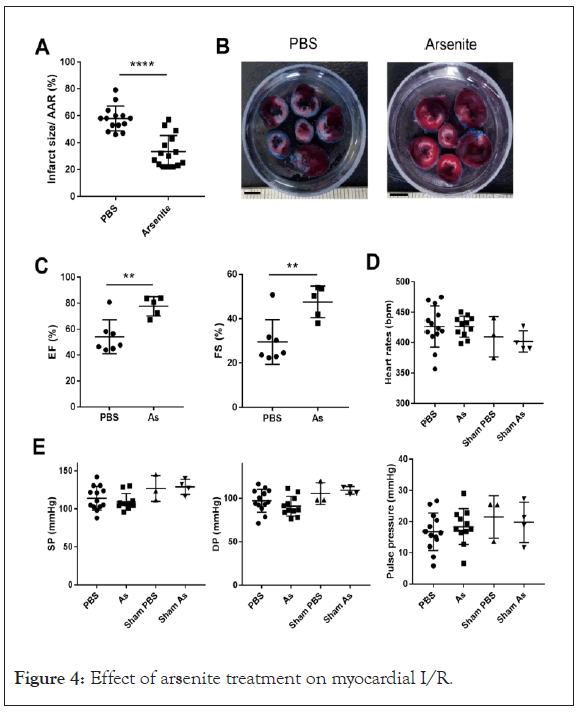
Figure 4: Effect of arsenite treatment on myocardial I/R.
(A) Quantification of infarct size of myocardial tissues 1 day after reperfusion (n=14 and 15) by TTC staining method. Rats were intraperitoneally administrated with PBS or 4.5 mg/kg arsenite 30 minutes before LCA was occluded. The data were analyzed using t-test, P<0.0001. AAR, the area at risk.
(B) Representative images of heart slices from different groups at 1 day after reperfusion. The non-ischemic area is indicated in blue, the area at risk in red, and the infarct area in white. Scale bars: 5 mM
(C) Ejection fraction (EF,%) and LV fractional shortening (FS,%) (n=5-7) analyzed by M-mode images of the LV from PBS and arsenite group 1 day after myocardial I/R. The data were analyzed using t-test, **P<0.01. As, Sodium Arsenite.
(D) and (E) The heart rates, Systolic Pressure (SP) and Diastolic Pressure (DP) were measured by placing a catheter into the femoral artery of rats at 24 hour after myocardial I/R. The pulse pressure was calculated by SP and DP. There is no significant statistic difference of these parameters in each group as sodium arsenite.
To determine the effect of arsenite on cardiac function after I/R, we measured Ejection Fraction (EF) and Fractional Shortening (FS) by echocardiography. The EF and FS were at a relative low level at 1 day after myocardial I/R in contrast with the reference, however, treatment with arsenite increased EF and FS after 1 day reperfusion, indicating improved cardiac function (Figure 4C). To test whether arsenite affects hemodynamic properties, we measured systolic pressure and diastolic pressure by placing a catheter into the femoral artery of rats at 24 hour after myocardial I/R. The heart rates, systolic pressure, diastolic pressure and pulse pressure were not significantly changed in each group (Figures 4D and 4E).
Arsenite induces a transient stress following sustained heme oxygenase-1 expression in myocardial tissue
Exposure to arsenite was shown to induce significant cellular stress, which is manifested as phosphorylation of eIF2α at Ser-51 and elevated expression of heat shock proteins, CHOP and GADD34 [14-20].
To explore the biological effects of arsenite on heart, cells or mice were simply treated with arsenite. Arsenite induced phosphorylation of eIF2α in rodent myocardial tissue and HeLa cells as early as in 1 hour (Figures 5A and 5B), which represented a cytosolic stress response is initiated. The mRNA level of stress related proteins such as CHOP and GADD34 was up-regulated transiently in heart tissue at 1 hour after peritoneal injection of arsenite (Figures 5B and 5C). HO-1, which protects cells from oxidative injury, was induced continuously at transcription level by arsenite in heart tissue but not in brain and liver tissue. HO-1 protein expression can be seen as early as 4 hours after arsenite treatment.
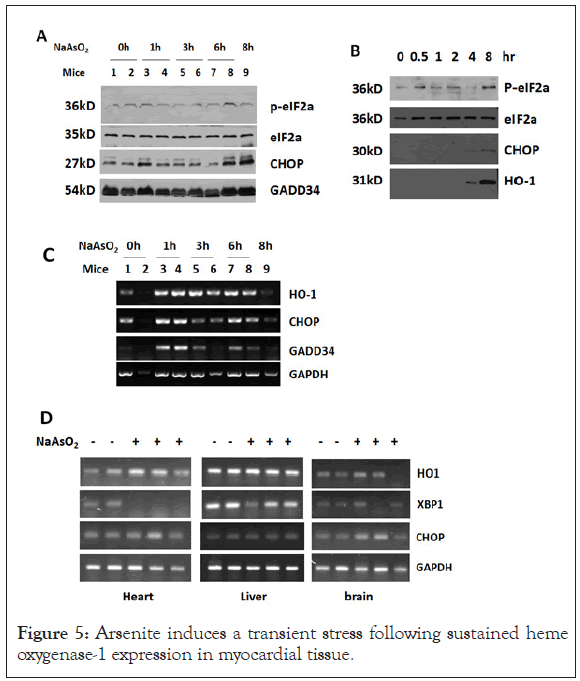
Figure 5: Arsenite induces a transient stress following sustained heme oxygenase-1 expression in myocardial tissue.
(A) Western blot analysis of total protein extracted from heart tissue of mice injected with 4.5 mg/kg sodium arsenite for the indicated time. (B) Western blot analysis of total protein extracted from HeLa cells treated with 4.5 μg/ml NaAsO2 for the indicated time. (C) RTPCR analysis of indicated genes in SMMC-7721 cells treated with NaAsO2 for the indicated times. (D) SD rats were intraperitoneally injected with 4.5 mg/kg NaAsO2 for 7 hours. After that total RNA was isolated from heart, liver and brain tissues and stress related genes were analyzed by RT-PCR.
The major findings of this study are as follows: (1) Arsenite blocks nigericin or Rotenone induced IL-1β secretion by inhibiting both the priming and activation stage; (2) Mitochondrial complex I respiration is inhibited by high dose arsenite; (3) Arsenite attenuates the myocardial ischemia-reperfusion injury in rats; (4) Arsenite induces HO-1 in myocardial tissue. However, the causal relationship among these activities needs further proof. The relationship between NLRP3 inflammasome and ischemiareperfusion injury has been studied for several years. Arsenite at least partially acts on myocardial tissue by this way.
Arsenite at 200 uM strongly inhibits oxygen consumption in isolated rat liver mitochondria with complex I–linked substrate. Whether arsenite targets the complex I or citric acid cycle needs further investigation. It’s reported that complex I switch between the active and de-active transition state [5]. In the context of IR injury, complex I readily undergoes deactivation during ischemia and is rapidly reactivated upon reperfusion. Deactive complex I exposes a critical cysteine residue (Cys39 in the ND3 subunit) that is occluded in active complex I. Importantly, covalent modification of Cys39 by thiol-reactive agents locks complex I in the deactive state preventing ROS production by RET. Trivalent arsenicals readily react in vitro with thiol-containing molecules such as GSH and cysteine.
Pyruvate dehydrogenase (PDH) is a multi subunit complex that requires the cofactor lipoic acid, a dithiol, for enzymatic activity. Arsenite inhibits PDH [27], perhaps by binding to the lipoic acid moiety. PDH oxidizes pyruvate to acetyl-CoA, a precursor to intermediates of the citric acid cycle. Accumulation of succinate is recently identified as a universal metabolic signature of ischemia in a range of tissues and is responsible for mitochondrial ROS production during reperfusion. The effect of arsenite on succinate level during ischemia is interesting.
Arsenite induces a cytosolic stress characterized by eIF2α phosphorylation and downstream events before myocardial ischemia. Pre and post conditioning with ischemia are representative strategies to reduce infarct size in animal models [28,29]. Both of these treatments in fact induce a cellular stress response, and arsenite may induce a similar cardiac stress response which is protective for the subsequent ischemia injury.
Heme oxygenase-1 and heat shock protein induction may contribute for cell survival during I/R. By degrading the oxidant heme and generating the antioxidant bilirubin and anti-inflammatory molecule carbon monoxide (CO), HO-1 protects cells from death due to path-physiological stress and oxidative injury. HO-1 has been shown to play a role in limiting myocardial Ischemia/Reperfusion injury in HO-1 knockout or transgenic mice. HO-1 protein level can be seen increased in 4 hours after arsenite treatment.
Citation: Li M, Mei Y, Liu J, Fan K, Gu X, Zhang X, et al. (2021) Inhibition of IL-1β Secretion and Mitochondria Respiration by Arsenite which acts on Myocardial Ischemia-Reperfusion Injury. J Clin Toxicol. 11:487.
Received: 28-Dec-2020 Accepted: 11-Jan-2021 Published: 18-Jan-2021 , DOI: 10.35248/2161-0495.21.11.487
Copyright: © 2021 Li M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.