Journal of Thermodynamics & Catalysis
Open Access
ISSN: 2157-7544
ISSN: 2157-7544
Research Article - (2012) Volume 3, Issue 3
A set of hydro desulfurization experiments are carried out on vacuum gas oil in a trickle bed reactor to model the effect of inhibition on the joint reactions of hydro desulfurization, and hydro denitrogenation. Dibenzothiophene (DBT) is used as a model compound in vacuum gas oil. In absence of nitrogen compounds over the tested catalysts in a trickle bed reactor at (523 to 573 K), 1 to 3 hr-1, and a pressure range of 16 to 20 bar the results show an increase in conversion when temperature increases, a little positive effect on conversions when pressure increases, and a decrease in conversion when LHSV increases.
Keywords: Hydrodesulfuization; Joint reactions; Inhibition; Trickle bed reactor
HDS of petroleum fractions is one of the most important processes in the petroleum industry to produce clean fuels. In particular, sulfur removal in diesel fuels is now strongly desirable for environmental and technical reasons. For instance, HDS is used to prevent atmospheric pollution by sulfur oxides produced during the combustion of petroleum-based fuels, to prevent poisoning of sulfur-sensitive metal catalysts used in subsequent reforming reactions and in the catalytic converter for exhaust emission treatment, finally, to avoid corrosion problems in engines [1]. The basic nature of these compounds causes them to adsorb onto Lewis acid sites on the catalyst surface, inhibiting the availability of the sites. This poisoning may be reversible or irreversible, depending on the HDS conditions [2]. High concentrations of organic nitrogen compounds can cause significant deactivation for reforming, cracking, hydro treating, or any other type of hydro processing catalysts [3]. The present work aims to study the inhibition effect of nitrogen compounds for HDS of DBT in VGO at using carbazole and acridine as non basic and basic compounds respectively over and prepared catalyst (PtMo/alumina) by finding inhibition factor and adsorption constant for each compound in a trickle bed reactor.
In all experiments, DBT was taken as a model compound with a constant concentration of 3000 ppm.
Materials and chemicals
The feedstock used in this study was non-hydrotreated VGO which was obtained from North Refineries Company. DBT was used as model sulfur compound while acridine and carbazole were used as model nitrogen compounds (basic and nonbasic). Hydrogen gas used for HDS of DBT while nitrogen used for purge oxygen from the system before any run. The catalyst ((0.5%) PtMo/Al2O3) was in this work, this type of catalyst was called Bimetallic catalysts which contains two active phase.
Catalyst preparation
Alumina was dried in oven at 393 K for 4 hours, this step was necessary to remove any moisture in the support before the impregnation, the active material chloro platinic acid was added to deionized water (Mo/ Al2O3 pore volume equal to deionized water volume) while the solution stirred for one hour at room temperature. Then the solution was filtered using a filter paper to get rid of precipitates, then the solution of active phase was added to Mo/Al2O3 at the rate of 15-20 drop per minute with continuous stirring until all the solution impregnated. The temperature was kept constant at 373 K through the impregnation by using a water bath. Then washing, drying and calcinations for 4 hours in the oven at 823 K was done.
Experimental procedure
The experiments were performed in a trickle bed reactor consisting of a 316 stainless steel tubular reactor, 77 cm long and internal diameter of 1.6 cm. The bed consisted of three main parts; two parts of glass rods and a part of catalyst which was loaded between them to provide plug flow conditions for isothermal reactions [4].
Figure 1 shows an experimental trickle bed reactor.
Laboratory tests
Catalyst characterizations: A D8 advanced X-Ray Diffractometer manufactured by Bruker AXS (Germany) was used to analyze crystallographic conditions of the bulk phase of catalyst samples. Data collection and manipulation was under automatically control of EVA software, which contains database of the JCPDS powder diffraction files and was operated under 40 kV and 40 mA and the scan was from 10° to 80° in a 2θ-angle with a scan speed of 0.04° per second. Scanning Electron Microscopy images of the catalyst surface are obtained using an high resolution FEI Quanta 200F field emission scanning electron microscope (FESEM) manufactured (United States) by with EDX system (INCA Energy 400) manufactured by OXFORD. To characterize the basic properties of catalytic samples, temperature programmed desorption (TPD) was used with carbon dioxide as the absorbate molecule, CO2 Temperature programmed desorption (TPD) experiments were carried out in a system supplied by Ohkura Riken Co. Ltd., (model TP-200). The equipment was developed to enable user to obtain data related to desorption characteristics of basic site present on catalyst. The Brunauer-Emmett-Teller (BET) surface area, pore volume and pore size measurements of catalysts were determined using Quanta Chrome Autosorb 6B system supplied by Quanta chrome Corporation (USA). The adsorption and desorption isotherms used in the evaluation of BET surface area were obtained at the boiling temperature of liquid nitrogen (78°K).
High Performance Liquid Chromatography (HPLC): DBT content in feedstock and product were determined using a computerize H.P.L.C DIONEX (UV (JYNKOTYK)/VIS160S), a C18 reverse phase column (Philips, 5 μm×0.4 cm). The mobile phase flow rate was 1 ml / min. DBT can be calculated by comparing the HPLC recorded area with calibration curves of DBT.
Reaction kinetics
The rate equation DBT conversion is divided into two stoichiometric equations:
1- Hydrogenlysis rate equation:
DBT + 2H2 → BiPh + H2S (1)
2- Hydrogenation rate equation:
DBT + 5H2 → CHB + H2S (2)
Numerous researchers concluded that hydrodesulphurization reaction is first order with respect to DBT concentration
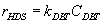 (3)
(3)
Where:kDBT is reaction rate constant, CDBT is concentration of DBT.
General stoichiometric equation reaction:
DBT + N-compound + H2 → H.C + H2S+ NH3 (4)
Where: N-compound is nitrogen compound, which is carbazole or acridine in the present work and H.C is a hydrocarbon.
Nitrogen compounds competitive with sulfur compound on the surface of catalyst, by substitution the fractional occupation of the DBT:
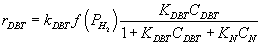 (5)
(5)
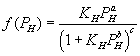 (6)
(6)
Sub. eq. (6) into equation (5):
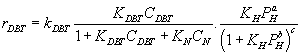 (7)
(7)
For first order reaction respect to hydrogen:
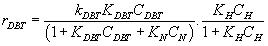 (8)
(8)
This equation is close to the general form of Langmuir-Hinshelwood.
Let: 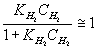 ,
, 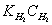
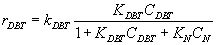 (9)
(9)
Neglect the self inhibition for DBT because the constant concentration for DBT therefore no self inhibition which meaning:
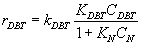 (10)
(10)
Let: 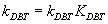 (11)
(11)
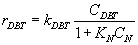 (12)
(12)
For non linear inhibition effect, Let (n) is exponent fitting the inhibition.
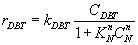 (13)
(13)
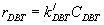 (14)
(14)
Where: rDBT is rate of reaction with inhibition of nitrogen compound, 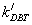 is rate reaction constant with inhibition.
is rate reaction constant with inhibition.
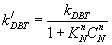 (15)
(15)
The inhibition features can be formulated as:
Inhibition effect
Inhibition effect represents by finding the (n) which is the fitting exponent for the inhibition effect of nitrogen compound.
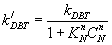 (16)
(16)
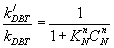 (17)
(17)
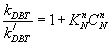 (18)
(18)
Adsorption equilibrium constant: The adsorption constant is an indicator of force to adsorption on the surface of catalyst with competitive of sulfur compound. When the adsorption constant is high that means, this nitrogen compound is high competitive with sulfur compound and have high adsorption on the surface of catalyst.
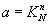 ,
, 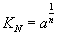 (19)
(19)
Reaction rate constant for inhibition: Reaction rate constant for inhibition can calculate from previous equation:
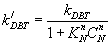 (20)
(20)
Inhibition factor: The inhibition factor (?) is a function of behavior nitrogen compound and show degree of inhibition, which increase when the initial concentration of nitrogen compound increase. The inhibition factor (?) can calculate from:
Inhibition factor 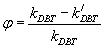 (21)
(21)
Catalyst characterizations
Figure 2 shows a good distribution of active metals (PtMo) which represent small particle size while the alumina support represent large particle or surface contain small particle.
The XRD pattern of homemade catalyst (PtMo) is given in the Figure 3. This figure indicates that calcination temperature does not change the crystalline structure of the catalyst. Figure 4 and Table 1 show the textural surface of homemade (PtMo) catalyst. The homemade catalyst has high surface area, so it has high macroporous particles and a low mesoporous particle, which means more voids between particles therefore an increase in pore volume of catalyst. It can be shown a reduce in surface area, pore volume, and pore size distribution after incorporation of platinum (Pt) for prepared catalyst, these results agree with Zden?ek Vít et al. [5] and Vishwakarma [6] who found a reduction of surface area for (Mo/alumina) deposited with different metals. Figure 5 shows the result of TPD for homemade PtMo, it is shown that there are no full peaks, so basic centers on the catalyst are little. Weak basic site on the hydrotreating catalyst is a very important point because these catalysts are acidic catalysts, for typical hydrotreating catalyst there are two functions, acidic support for cracking heteroatom bonds and metals for hydrogenation [7,8]. So the strength of hydrotreating base to acidity result show that the strength of basic very weak and strength of acidic very high, therefore these result are very excellent.
| Property | Method | Mo | PtMo | CoMo |
|---|---|---|---|---|
| Total surface area, m2/gm | BET | 265.7 | 224.3 | 184.6 |
| Pore volume, cm3/gm | BET | 0.73 | 0.64 | 0.43 |
| Average pore diameter, A | BJH | 56.72 | 67.24 | 98.37 |
Table 1: Texture properties of commercial and prepared catalysts.
Inhibition effect
Inhibition effect can be represented by exponent factor (n) which is a power of inhibition effect for nitrogen compound. For the present study (n) is found by fitting the nitrogen compound data, the procedure is shown in the Figures 6-9. The adsorption constant for carbazole and acridine can be calculated by equation (20). The adsorption constant for carbazole is (367.87) at a concentration range of (0-200 ppm) over (PtMo) catalyst respectively at 20 bar, 1 LHSV and 573 K. The adsorption constant for acridine is (564.537) at concentration (0 to 200 ppm) over (PtMo) catalyst respectively at 20 bar, 1 LHSV and 573 K. At large value of adsorption constant nitrogen compound is highly competitive with sulfur compound and highly adsorbed on the catalyst, also when the adsorption constant is low nitrogen compound, it exhibit low competition with sulfur compound and weakly adsorbed on the catalyst surface. These results agree to Laredo (2003) [9] who found that the adsorption constant for carbazole is (328.2), indole (1834.9) and quinoline (661.2) also Laredo et al. [10] and Laredo et al. [11].
Inhibition factor
Figures 10-12 show the inhibition factor for carbazole and acridine over PtMo catalyst. The inhibition factor of basic nitrogen compound is more than that of non basic for both catalyst, the results show that the inhibition factor for carbazole is (0.537) over PtMo for inhibition of 100 ppm of carbazole, while inhibition factor for carbazole is (0.642) over PtMo catalyst for inhibition of 200 ppm. The results show that the inhibition factor for acridine is (0.608) over PtMo for inhibition by 100 ppm acridine, while the inhibition factor for acridine is (0.708) for inhibition by 200 ppm. These results agree with those of Laredo (2003) [11].
Activation energy
According to Arrhenius equation, a plot of (ln K) versus (1/T) gives straight line with slope equal to (-E/R), from which the activation energy is calculated as illustrated in figure 13. The obtained values of activation energies are presented in Table 2. Farag [12] found activation energy of DBT over two different types of catalyst MoS2, CoMo/Al2O3 at same conditions is (79.002), (43.89) KJ/mol respectively. Secondly the type of solvent, Kabe et al. [13] found activation energy of DBT in two different types of light gas oil over same type of catalyst CoMo/Al2O3 at the same conditions and is (108.68), (112.86) KJ/mol respectively. Thirdly is the type of sulfur compound, activation energy for total sulfur almost higher than individual, Steinner and Blekkan [14] found that the activation energy for total sulfur of light gas oil is (119.966) KJ/mol depending on contains of sulfur compound in oil. For present study these results disagree with Kabe et al. [15] who found (100.32) KJ/mol for DBT, this comparison with the same individual sulfur compound.
| Researcher | Activation eng. | Catalyst | Sulfur type |
|---|---|---|---|
| Steinner2002 | 119.966 | NiMo | Total S in LGO |
| Laredo2003 | 112.86 | NiMoP | DBT in diesel feed |
| Vít 2005 | 63 | PtMo | Thiophene |
| Vít 2005 | 76 | CoMo | Thiophene |
| Farag 2007 | 43.89 | CoMo | DBTin decane |
| Waleed 2010 | 81.067 | CoMo | DBT in VGO |
| Waleed 2010 | 68.391 | PtMo | DBT in VGO |
Table 2: Comparison present activation energy with other studies.
The resultant models
Kinetic models without inhibition: First order kinetic reaction respect to DBT concentration. Reaction rate equation:
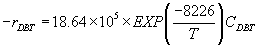 (22)
(22)
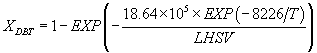 (23)
(23)
HDS of DBT inhibited by carbazole and acridine over PtMo: Non linear inhibition, fitting exponent n=0.5 for carbazole and acridine, reaction rate equation with inhibition:
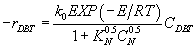 (24)
(24)
With inhibition over PtMo: 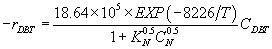 (25)
(25)
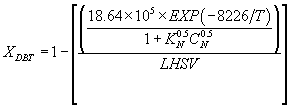 (26)
(26)
1) HDS of DBT present in VGO is highly dependent on temperature and liquid hourly space velocity variation at constant concentration of DBT under specified conditions; pressure range 16 to 20 bar, temperature range 523 to 573K, liquid hourly space velocity range 1 to 3 hr-1, concentration of DBT in VGO constant 3000 ppm.
2) The study of inhibition of HDS of DBT by non basic nitrogen compound carbazole and basic nitrogen compound acridine showed a highly non linear inhibition depends on the concentration of nitrogen compound, inhibition effect of basic nitrogen compound acridine more than non basic nitrogen compound carbazole for all conditions.