Clinical Pediatrics: Open Access
Open Access
ISSN: 2572-0775
ISSN: 2572-0775
Research Article - (2024)Volume 9, Issue 6
In sickle cell disease cardiomyopathy, 3D transthoracic Echocardiography (3D Echo) is an essential diagnostic tool for accurate diagnosis and further understanding of its pathophysiology. To this end, we performed a retrospective observational study in a cohort of 46 pediatric patients with homozygous or heterozygous sickle cell disease. In particular, we assessed that an increased right ventricular end-diastolic volume is the earliest sign of this disease progression, promoted by a high pulmonary artery systolic pressure and followed by uncoupling with the pulmonary artery. Over time, the dysfunction of the right ventricle also affects the left ventricle, leading to global heart failure, which can be considered "right ventricle-driven". In addition, 3D echocardiography is an essential tool in the follow- up of this disease and together with the reduced incidence of acute chest syndrome or peripheral vaso-occlusive events to choose the optimal medical treatment.
Sickle cell disease; Pediatric cardiomyopathy; Pediatric 3D echocardiography; Pediatric cardiomyopathy Pathophysiology; Right ventricle failure
Sickle Cell Disease (SCD), as a multisystemic genetic disorder in both its dominant and recessive forms, has a profound effect on the cardiovascular system, greatly influencing the prognosis of each individual case [1]. The cardiovascular aspect of this disease is particularly relevant in today's era of sophisticated imaging tools that allow to define pathophysiological correlations, otherwise unclear [2]. Our observational retrospective study addressed this issue by reviewing 3D Echo images.
Our research included a group of 46 black children, immigrated to Italy from Sub-Saharan countries and observed from 2019 to the present, 24 males, aged between 3 and 12 years, with an average age of 7 years. 32 children were homozygous (HbSS), of which 5 were HbSB0, while the other 12 were heterozygous (HbSC). According to our protocol, they underwent an annual check-up for the most important laboratory tests and a 3D Echo every 6 months, performed with an angle-independent, less load-dependent and highly reproducible machine. In particular, we formally considered relevant to study the left atrium, Left Ventricular (LV) End-Diastolic Volume (LVEDV), its Ejection Fraction (LVEF) and Global Longitudinal Strain (LVGLS), measuring the corresponding parameters. In addition, we carefully assessed the Right Ventricle (RV) Ejection Fraction (RVEF), End Systolic Volume (RVESV), End Diastolic Volume (RVEDV) and Free Wall Longitudinal Strain (RVFWLS), combining three- and four-chamber projections, potential recognition in the 3D Echo technology. 22 children (47%) had undergone medical treatment with hydroxyurea, which, regardless of genotype, reduced the total incidence of acute chest crises to 2 per year in 8 patients and to 1 per year in 14 others. The 3D Echo results were matched with the body surface area and mass index to confirm their real impact [3]. The Ethics Committee of our institution, the University of Modena and Reggio Emilia, confirmed that this study did not require ethical approval, for its retrospective nature, nor parenteral consent, giving that the procedures performed were part of the routine care.
All children, if examined in a stable condition, showed normal metabolic parameters with the exception of a slight increase in Lactate Dehydrogenase (LDH) and unconjugated bilirubin. Anemia, a leading sign of SCD, varied between 8.25 g/dl and 13.87 g/dl, with a mean value of 10.37+1.44, a corresponding increase in reticulocytes, but normal polymorphonuclear and platelet counts. The standard 3D Echo LV indexed parameters were within the normal range (Table 1). On the contrary, the only pathological parameters concerned RV, in particular an early increased Pulmonary Artery Systolic Pressure (PASP) s, more frequent in homozygous than in heterozygous children (55% versus 33%). Its average progression was proportional to age, varying from 10% to 30% in 5 years and associated with a corresponding increase in RVEDV and a decrease in RVFWLS, observed in cases with a >20% increase in PASP. This adverse clinical course mainly affected children with extra-cardiac SCD complications, homozygous state, lower efficacy of or adhesion to pharmacological treatment and correspondingly higher incidence of episodes of acute chest crisis and vaso-occlusive events.
| Characteristic | Mean ± SD | Range |
|---|---|---|
| LVEF % | 57.41 ± 3.24 | 52.00 to 65.00 |
| LVESV ml | 38.9 ± 9.54 | 21.20 to 59.75 |
| LVEDV ml | 88.9 ± 20.76 | 54.55 to 140.89 |
| LVGLS % | -19.94 ± 3.00 | -25.00 to -13.50 |
| RVEF % | 55.70 ± 2.28 | 51.60 to 60.70 |
| RVESV ml | 60.74 ± 37.69 | 28.30 to 132.90 |
| RVEDV ml | 127.27 ± 92.88 | 28.60 to 306.20 |
| RVFWLS % | -24.56 ± 6.23 | -32.60 to -6.50 |
Note: SD: Standard Deviation
Table 1: Indexed 3D-echocardiographic results.
Given the small number of patients studied, their wide age range and variable impact of possible balancing factors, we considered it inappropriate to statistically re-estimate our results. Similarly, microvascular factors, the common background of SCD, are difficult to assess quantitatively. On the contrary, cardiac 3D Echo morphological features are directly suitable to be deepened from a pathophysiological point of view. In any case, the primary role of a high PASP in promoting RV damage and consequent chain of adverse cardiac events is directly evident. Similarly, the possible interference of other, as yet unknown, humoral factors may be considered as a link between a microvascular pathology and negative cardiac events [4].
Microvascular pathology
At a microvascular level, chronic anemia causes peripheral vasodilation and increases circulating blood volume through the secondary activation of the renin-angiotensin-aldosterone system. As a result, blood viscosity and peripheral resistances decrease, while blood flow velocity and shear stress on capillary walls increase, followed by abnormal release of von Willebrand or other coagulation factors [5-7]. In addition, the augmented intravascular lysis of SCD erythrocytes and activation of leukocytes and platelets stimulates release of interleukins and generation of cell-derived particles [8]. All these factors, combined with a reduced bioavailability of nitric oxide and greater production of nitrogen dioxide and endothelin-1 factor, favor diffuse microthromboses [9,10]. This pathology electively affects the pulmonary microvasculature, firstly by promoting arterioles contraction and consequently their wall hypertrophy through proliferation of medial smooth muscle cells, a key component of irreversibly increased PASP. Another ubiquitous element is increased tissue deposition of bioactive iron, including myocardial tissue [11].
Cardiac pathology
In addition to this complex chain of microvascular events, cardiac function is impaired by other correlated mechanisms, namely tachycardia and hyper-dynamic circulation. They cause a proportional lengthening of the RV myocardial fibers, useful within the limits of the Frank-Starling law. However, beyond a physiological threshold, this process becomes detrimental and leads to a progressive spherical remodeling of the RV and a parietal concentric hypertrophy, which increases the RVEDV and shifts its contraction towards a horizontal or semi-horizontal plane, at the expense of the more physiological contraction along the longitudinal axis [12-14]. In addition, within the pericardium, the augmented RVEDV and consequently internal tension, typical in the late diastolic phase, opposes the LV diastolic compliance. This effect, proportional to the increased circulating blood volume, simulates a restrictive cardiomyopathy with paradoxically preserved ejection fraction (Figure 1) [15,16]. On 3D Echo imaging it correlates with a D-shaped Interventricular Septum (IVS) protruding into LV and a right atrioventricular plane pushed up towards the right atrium, causing an abnormal systolic excursion of the tricuspid annulus, in turn predisposing to a tricuspid valve insufficiency. A further aggravation consists in a progressive RV-pulmonary artery uncoupling [17,18]. As a countermeasure, this negative hemodynamics, electively damaging the RV, activates a support from the LV, through interdependent anatomical structures. These consist of myocardial fibers extending from the inner walls of the RV through the IVS to the LV sub-epicardium [19-25]. Embryologically, this correlates with the common origin of both the ventricles from a single primordial structure [26,27]. In absence of arrhythmias, this cardiovascular system can be compared to an electric circuit with two secondary branches derived in "parallel", to which, according to Newton's second law and Ohm's principle, energy is supplied proportionally to their capacity and need. However, despite all these protective mechanisms and possible benefits of medical treatment, a progressively augmented PASP can increase RV afterload and RVEDV (Figure 2). Consequently, in the long term, the LV, already damaged by chronic anemia, abnormal tissue deposition of bioactive iron, persistently elevated inotropic state and possible endothelial dysfunction of its coronary microvasculature, may become progressively insufficient and through an early augmented left atrium inner tension, increase pulmonary microvascular resistance. This condition may lead to a global heart failure [28-30]. It can be considered "RV-driven", given the frank predominance of the RV pathology (Figure 3). It can be hypothesized that an increased diastolic pulmonary artery pressure, which cannot be assessed in 3D Echo explorations, may correspond to an increased RV inner tension and RVEDV [31-37].
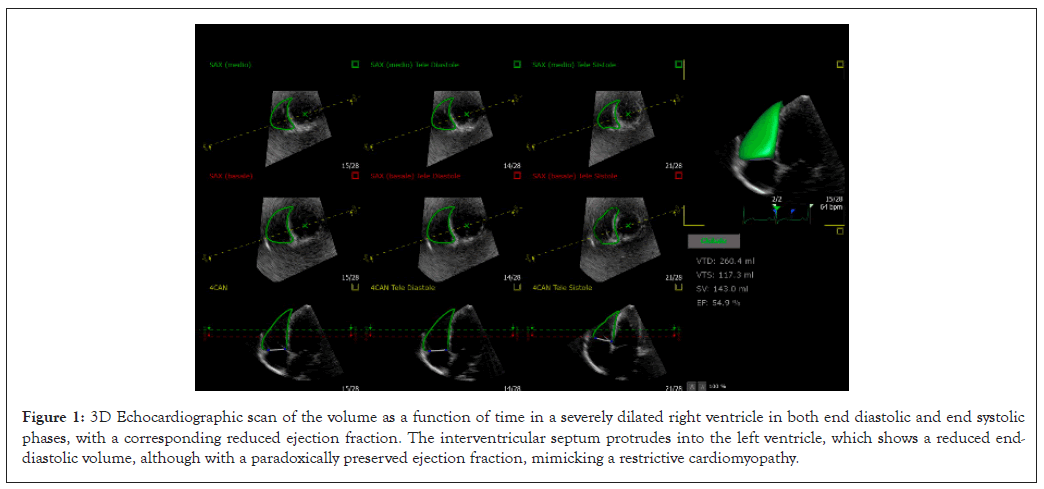
Figure 1: 3D Echocardiographic scan of the volume as a function of time in a severely dilated right ventricle in both end diastolic and end systolic phases, with a corresponding reduced ejection fraction. The interventricular septum protrudes into the left ventricle, which shows a reduced end- diastolic volume, although with a paradoxically preserved ejection fraction, mimicking a restrictive cardiomyopathy.
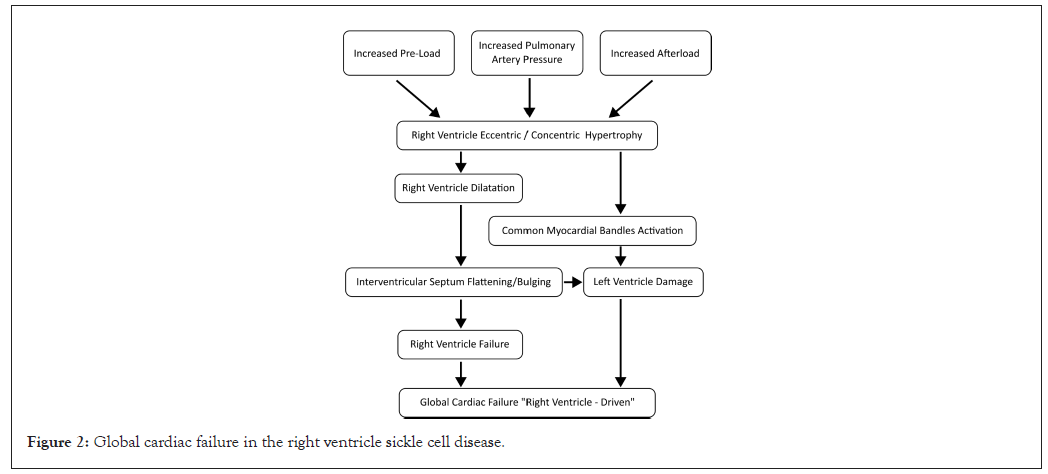
Figure 2: Global cardiac failure in the right ventricle sickle cell disease.
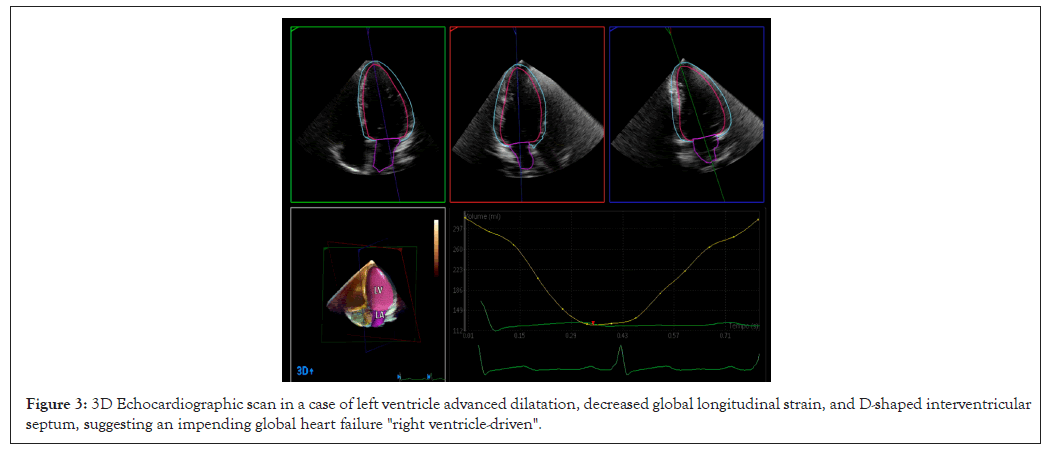
Figure 3: 3D Echocardiographic scan in a case of left ventricle advanced dilatation, decreased global longitudinal strain, and D-shaped interventricular septum, suggesting an impending global heart failure "right ventricle-driven".
The current 3D Echo technology has a primary role in diagnosis, follow-up and deepening pathophysiological characteristics of SCD cardiomyopathy, in particular of the RV. Corresponding results can be obtained by Magnetic Resonance Imaging (MRI) contrast enhanced, mainly if 4D formatted, however technologically more demanding. Equally, multi-center clinical studies may be proposed, possibly performed with artificial intelligence methods, in order to collect and examine a greater number of clinical data. Practically, detection of RV overload or PASP progressive increase, alert to an early cardiac insufficiency and a more appropriate medical treatment.
No conflict of interest declared.
FC: Carried out data collection. FT: Wrote the first draft of the manuscript. GP: Reviewed clinical cases. BM: Guided interpretation of the results. GP: Interpreted the result. FF: Performed data research. AM: Wrote manuscript; AVM: Corrected manuscript; LI: Overview of text; GB: Interpreted pathophysiology.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Coppi F, Tampieri F, Palazzi G, Boschini M, Pagnoni G, Ferrara F, et al. (2024). Insights into Pathophysiology of Sickle-cell Disease Cardiomyopathy by 3D Echocardiography. Clin Pediatr. 9:278.
Received: 21-Oct-2024, Manuscript No. CPOA-24-34330; Editor assigned: 23-Oct-2024, Pre QC No. CPOA-24-34330 (PQ); Reviewed: 06-Nov-2024, QC No. CPOA-24-34330; Revised: 13-Nov-2024, Manuscript No. CPOA-24-34330 (R); Published: 21-Nov-2024 , DOI: 10.35248/2572-0775.24.9.278
Copyright: © 2024 Coppi F, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.