Journal of Cell Science & Therapy
Open Access
ISSN: 2157-7013
ISSN: 2157-7013
Review Article - (2021)
Behavioral plasticity is one of the most important strategies by which animals can adapt to transient environmental changes for their survival. Biological systems must be flexible enough to induce and maintain behavioral plasticity while still being finely regulated, especially in response to life-threatening situations like starvation. Animals produce behavior in response to stimuli, which can be altered when starvation is paired with a range of stimuli (associative learning). Such mechanisms of associative learning have been studied extensively in C. elegans. Use of C. elegans provides an ideal system to study the neural mechanisms of integration of external cues with internal state for multiple reasons. First, C. elegans is one of few organisms for which the complete, stereotyped connectome of neurons is available. This allows researchers efficiently identify the responsible neural circuit for associative learning with stimuli such as odor, salts, and temperature. Secondly, although worms have much simpler structure than higher organisms, genes and signal cascades are surprisingly well conserved. One of the evolutionally conserved signaling pathways, the insulin signaling pathway, plays an important role in nervous system of worms to integrate starvation signaling with environmental cues. This review highlights the recent findings on the function of insulin signaling in starvation-associated behavioral plasticity in C. elegans.
C. elegans; Insulin signaling; Starvation; Learning; Plasticity
Note: AFD=A pair of the major thermosensory neurons in C. elegans; AGE-1/age-1= C. elegans homolog of PI3K; AIA, AIY, AIZ=Pairs of first layer interneurons of C. elegans ; AKT=Serine/threonine kinase in insulin signaling pathway; AMPA=Type of glutamate receptor in mammalian hippocampus; ASE(R/L)=(Right/Left) neuron of a saltsensory neuron pair; ASI=A sensory neuron pair that responds to various stimuli including temperature and salts; AWC=Sensory neuron that responds to various stimuli including temperature and attractive odors; CASY-1=Homolog of calsyntenins; C. elegans= Caenorhabditis elegans; Nematode; worm, commonly used as a model animal; DAF-2=C. elegans homolog of the mammalian insulin receptor; DAF-16=C. elegans homolog of FOXO; DAF-18/daf-18=C. elegans homolog of PTEN; EPIL=Placenta insulin-like peptide; FOXO=Family of forkhead box O transcription factors; IGFs=Insulin-like growth factors; INS-1=C. elegans ortholog of mammalian insulin; KLC-2=Kinesin Light Chain; NLP-1=Neuropeptide involved in olfactory learning in C. elegans; PDK-1=Kinase in insulin signaling pathway; PI3K=PI3-kinase=Phosphoinositide 3-kinase, kinase downstream of the insulin receptor in the insulin signaling pathway; PIP3=Intermediate phospholipid in insulin signaling pathway; PTEN=Phosphatidylinositol-3,4,5-trisphosphate 3-phosphatase. Identified as a tumor suppressor, but has various Journal of Cell Science & Therapy Review Article Correspondence to: Asuka Takeishi, RIKEN Center for Brain Science (CBS), Wako, JAPAN, E-mail: asuka.takeishi@riken.jp Received: August 20, 2021; Accepted: September 03, 2021; Published: September 12, 2021 Citation: Galatsis KN, Takeishi A (2021) Insulin Signaling Acts Extensively in C. elegans Starvation-Associated Learning and Behavioral Plasticity. J Cell Sci Therapy.S5:315. Copyright: © 2021 Galatsis KN, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. J Cell Sci Therapy, Vol.12 Iss.S5 No:1000315 1 functions; Ras-MAPK=Signal transduction pathway; RIA=interneuron and part of core thermotactic circuit Tc=C. elegans cultivation temperature
The ability to modify behavioral output based on internal state is crucial for the survival of an organism. It allows hungry animals to devote more resources to food acquisition, sexually mature individuals to prioritize finding a mate, and other behavioral modulations that serve to increase the survival possibilities of individuals and the species. Among these internal states, animal response to satiety is especially wellcharacterized: Animals exhibit feeding-state dependent responses to environmental stimuli [1,2]. In addition to the core neural network responsible for sensation of specific environmental stimuli, multiple neurons are implicated in the homeostatic regulation of hunger in order to drive foraging behavior [3].
C. elegans is an organism well suited to dissect how feeding-state modulates neural circuit dynamics since the complete connectome of their 302 neurons is characterized [4,5]. This connectome is extremely stereotyped, and both neuron number and connections between neurons in the whole animal remain standard across individuals. Despite their simple nervous system, C. elegans have been shown to display various types of learning, including odor-based, salt-based, and temperature-based learning [6-8]. Additionally, C. elegans is transparent allowing researchers to perform live-imaging on intact animals that express florescent proteins as well as single-cell ablation experiments to be conducted by sight.
When C. elegans are exposed to naturally attractive chemicals such as odorants and salts during starvation, they learn to avoid the paired chemical. INS-1/insulin has been identified as a key regulator of such starvation-associated behavioral plasticity. INS- 1/insulin is a secreted signaling molecule and can mediate systemic communication of feeding state to its target cells, including the nervous system [7,9,10]. Recent work has uncovered the systemic mechanism by which INS-1/insulin signaling regulates starvation-dependent impairment of thermotaxis behavior. INS-1/insulin from the gut acts on the chemo/thermosensory neuron AWC and modulates AWC activation to induce suppression of thermotaxis toward colder temperatures (negative thermotaxis) [11].
In this review, we describe how and where INS-1/insulin signaling acts in the nervous system of C. elegans to regulate starvation-associated learning and behavioral change. Upon transient alteration in feeding-state, the INS-1/insulin signaling pathway is able to induce specific behavioral changes by effectively recruiting various cell types, including neurons and gut. This flexible but well-controlled neural dynamics likely allow worms to adapt their foraging strategy precisely to maximize their survival.
Overview on insulin signaling pathway
The superfamily of insulin and its related proteins regulate a ubiquitous signaling transduction in vertebrates and invertebrates, including C. elegans [12-15]. Insulin is a hormone well known in humans for regulating the uptake of glucose into cells. In addition to insulin, the insulin superfamily in humans comprises Insulin-like Growth Factors (IGFs), relaxins, Early Placenta Insulin-like Peptide (EPIL), and the relaxin-like factor [16-19]. This collection of hormones acts broadly in the body and serves a variety of functions: IGFs stimulate mitosis and mediate growth and division in cells, while relaxins have been shown to act in vasodilation [20]. However, the complete functions of the superfamily members in each tissue remain to be fully elucidated.
The canonical insulin signaling pathway is triggered by insulin receptor activation in response to the binding of insulin. Activated insulin receptors send a signal to Phosphoinositide 3- Kinase (PI3K) that in turn produces phospholipid products such as PIP3, that act on serine/threonine kinases PI3K-AKT (Figure 1). PI3K-AKT then phosphorylates downstream targets. Recent studies identified that insulin signaling modulates gene expression via the Forkhead Box O (FOXO) family of transcription factors (Figure 1) [21].
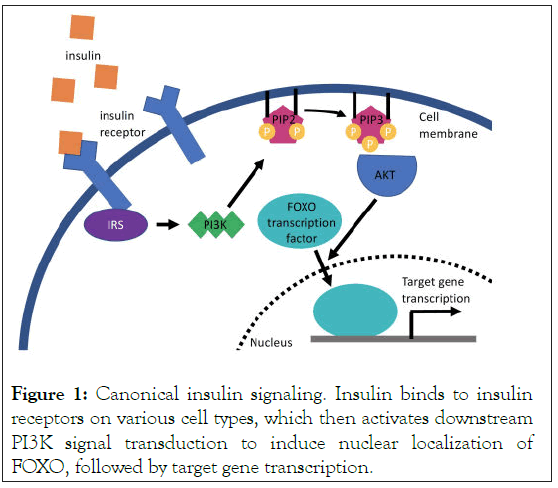
Figure 1: Canonical insulin signaling. Insulin binds to insulin receptors on various cell types, which then activates downstream PI3K signal transduction to induce nuclear localization of FOXO, followed by target gene transcription.
The insulin signaling pathway is also important in the nervous system of mammals to integrate internal information for proper behavior. Insulin receptors are widely expressed in the rat brain [22]. Insulin receptors that localized to the post-synaptic region stimulate AMPA receptor endocytosis in hippocampal CA1 neurons resulting in long- term depression of the synapses [23,24]. Also, inhibition of insulin receptors in the central nervous system results in learning and memory defects [25]. More specifically, inhibition of PI3K, a kinase in the insulin signaling pathway, results in diminished insertion of AMPA receptors into the synapse during long-term potentiation in the hippocampus [26].
40 insulin-like peptides (The ins gene family) were identified in C. elegans including an ortholog of human insulin, INS-1 Pierce, et al. [27,28] (Figure 2). Despite these numerous signaling molecules, there is only a single homolog of the insulin receptor, DAF-2 in C. elegans [29]. DAF-2 is expressed in many cells in the worm including head neurons, intestine, and others. Activation of DAF-2/insulin receptor follows the general signaling cascade similar to mammals, resulting in the expression change of target genes via DAF-16, a single ortholog of FOXO (Figure 2). The insulin pathway of C. elegans has been suggested to regulate various functions such as lifespan, thermotaxis behavior, hypoxia sensitivity, and salt learning plasticity [7,30-32]. It also mediates a stress response unique to C. elegans called dauer larva formation: A form of stress-resistant developmental arrest that can be induced in the presence of adverse conditions such as starvation, high temperature, or overcrowding [31].
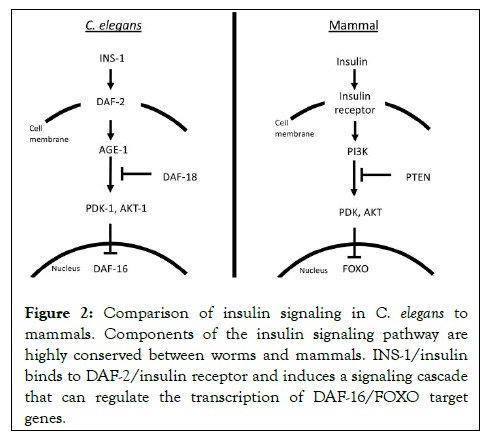
Figure 2: Comparison of insulin signaling in C. elegans to mammals. Components of the insulin signaling pathway are highly conserved between worms and mammals. INS-1/insulin binds to DAF-2/insulin receptor and induces a signaling cascade that can regulate the transcription of DAF-16/FOXO target genes.
Here, we would like to focus on the function of INS-1 and discuss its function on learning and behavior regulation of C. elegans.
Insulin signaling in olfactory learning
Olfactory plasticity is one of the more studied mechanisms of behavior plasticity in worms, with olfactory adaptation being described genetically over 20 years ago [6]. The general learning paradigm used in olfactory plasticity experiments is the same classical conditioning first described in mammals and later used in Drosophila, demonstrating a conservation of associativelearning strategies among the species [33,34].
Sensory neurons in the head and tail of the worm detect odorants in the environment and induce chemotaxis behavior: Worms migrate toward attractive compounds and away from unattractive ones (Figure 3A). Worms are naturally attracted to or avoid certain volatile compounds and salts, but their chemotaxis toward these chemicals is often altered based on experiential learning. For instance, benzaldehyde is a naturally attractive odor to C. elegans, but when paired with starvation the worms will learn to avoid it [35]. Worms carrying mutations in the insulin signaling pathway were shown to exhibit severe defects in benzaldehyde-starvation learning [9]. Increased insulin signaling in worms “flip a switch” in sensory neuron AWC, that turns naïve attraction to benzaldehyde into repulsion. This behavior switching is mediated possibly by increasing the synaptic integration of DAF-2/insulin receptors or the activity of its downstream molecules [9].
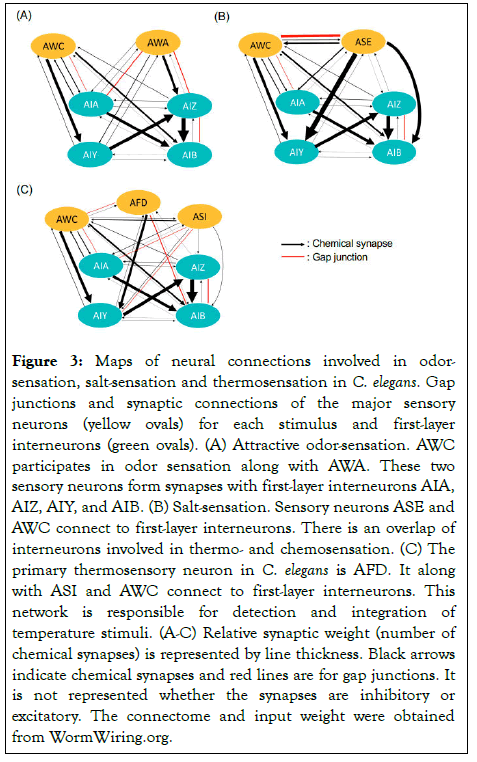
Figure 3: Maps of neural connections involved in odorsensation, salt-sensation and thermosensation in C. elegans. Gap junctions and synaptic connections of the major sensory neurons (yellow ovals) for each stimulus and first-layer interneurons (green ovals). (A) Attractive odor-sensation. AWC participates in odor sensation along with AWA. These two sensory neurons form synapses with first-layer interneurons AIA, AIZ, AIY, and AIB. (B) Salt-sensation. Sensory neurons ASE and AWC connect to first-layer interneurons. There is an overlap of interneurons involved in thermo- and chemosensation. (C) The primary thermosensory neuron in C. elegans is AFD. It along with ASI and AWC connect to first-layer interneurons. This network is responsible for detection and integration of temperature stimuli. (A-C) Relative synaptic weight (number of chemical synapses) is represented by line thickness. Black arrows indicate chemical synapses and red lines are for gap junctions. It is not represented whether the synapses are inhibitory or excitatory. The connectome and input weight were obtained from WormWiring.org.
Even when starvation is not paired with a conditioned odor stimulus, insulin signaling acts on the chemosensory circuit to guide foraging behavior [10,36]. When worms are removed from food and allowed to forage, wild-type worms increase their turning behavior (local searching) within initial 15 minutes. After the 15 minutes of food removal, this local searching is attenuated in favor of longer-range random walking biased by available cues (odors, salts, etC.). Mutants for neuropeptide NLP-1 showed increased turning compared to wildtype during local searching period but had no defects in suppressing turning behavior once the time period elapsed. NLP-1 mediates a neuropeptide feedback loop between AWC and AIA: Release of NLP-1 from AWC causes AIA to release INS-1/insulin that acts either directly or indirectly to dampen AWC calcium transients. As AWC activation induces turning behavior, NLP-1 secretion ends up inhibiting AWC activity and turning behavior. Insulin signaling thus can bias locomotor behavior toward more favorable foraging strategies during prolonged removal from food.
Insulin signaling in chemotaxis to salt
The insulin signaling pathway has been shown to regulate plasticity of salt chemotaxis in C. elegans as well [7]. Worms that have not undergone any training migrate toward NaCl (positive chemotaxis) while worms that have experienced NaCl paired with starvation will lose attraction to the salt (starvationconditioning) [37,38]. This learning depends on PI3-kinase activity and is altered in insulin signaling pathway mutants [7]. Double mutants for age-1/PI3-kinase; daf-18/PTEN homolog show no PI3-kinase activity, and only a mild decrease in NaCl chemotaxis is observed after salt-conditioning [7]. The ASE neuron pair is responsible in salt sensation in C. elegans (Figure 3B). Upon starvation, the right-side neuron, ASER, receives INS-1/insulin secreted by the AIA interneurons, activate DAF-2/insulin receptor and downstream signaling, and phosphorylates AKT-1 and PDK-1 [7,39]. Phosphorylation of these downstream kinases is responsible for modification of sensory function in ASER by starvation-conditioning that results in the reversal of the preference for NaCl.
Through use of a starvation-dependent salt avoidance assay, the details of insulin-pathway regulation were revealed [40]. A novel isoform of the DAF-2 receptor, DAF-2c, was identified and shown to localize to the axon in ASER when worms were starved. Axonal localization of DAF-2c increased salt avoidance through subcellular activation of PI3K. Synaptic translocation of DAF-2c is mediated by Ras-MAPK pathway that acts via CASY-1 (homolog of calsyntenins) and KLC-2 (kinesin light chain). Enhanced salt aversion is observed even in the presence of food in Ras-MAPK pathway mutants. Mutants that diminish Ras MAPK signaling increase insertion of DAF-2c in the axon, mimicking starvation, which confirms ASER activity confers behavioral plasticity under starved conditions.
Insulin signaling in thermotaxis
Thermotaxis of C. elegans is unique because when worms are well fed, they memorize their cultivation temperature (Tc), move toward it, and track isotherms around Tc [8]. In aversive conditions, such as starvation or overcrowding, worms lose this preference for Tc [8]. Thermotaxis is thus produced by associating temperature with either favorable or unfavorable conditions. Laser ablation of individual neurons in live animals has identified AFD as the major thermosensory neuron and AIY and AIZ as responsible for thermophilic and cryophilic movement respectively (Figure 3C) [41]. While AFD is the primary temperature sensing neuron, later work has shown AWC and ASI to play a role in temperature sensation (Figure 3C) [42-44]. Within the circuit, AFD, AWC, and ASI synapse onto AIY, which itself synapses onto AIZ and RIA (Figure 3C). AIZ also synapses directly onto RIA, and the balance of input from the interneurons drives thermotaxis behavior [45].
INS-1/insulin again acts as a key modulator that integrates information of feeding state with temperature [46]. INS-1/ insulin mutants do not show starvation- dependent thermotaxis plasticity, and the plasticity defect was partially rescued by mutations in DAF-2/insulin receptor or AGE-1/PI3-kinase [46]. It suggests that INS-1/insulin functions antagonistically to DAF-2/insulin receptor, opposite in function to associativelearning of chemotaxis in C. elegans and in humans/mammals where insulin is purely agonistiC. It is still unclear how INS-1/ insulin can function bidirectionally on DAF-2/insulin receptor in C. elegans. The direction of INS-1/insulin on DAF-2/insulin receptor might depend on which isoform of DAF-2/insulin receptor (e.g., DAF-2c) receives ins-1 signaling or/and subcellular localization of DAF-2/insulin receptor as mentioned above.
Recent work also offers a mechanistic view of insulin signaling in thermotactic plasticity [11]. Interestingly, despite the suppression of negative thermotaxis (migration toward the colder side) under starvation, calcium responses of core thermosensory neurons, AFD and AIY, are largely unchanged [11,47]. Rather, starvation-dependent negative thermotaxis attenuation is mediated by alteration of AWC-AIA activities, hyperactivated and inhibited respectively, by starvation. AWC likely receives INS-1/insulin signaling from the gut upon starvation, which induces depolarization of itself and activation of DAF-16/FOXO transcription factor [11]. These results indicate that worms recruit AWC-AIA circuit through the insulin signaling pathway only when they are starved to induces thermotaxis plasticity. This efficient mechanism of statedependent neuromodulation by altering circuit dynamics might allow C. elegans to precisely tune their behavior even with their simple nervous system.
Insulin signaling in maintenance of behavioral plasticity during aging
Genetic manipulations that suppress the insulin signaling pathway have resulted in consistently extended lifespan in various organisms including humans, mice, flies and worms [48]. Additionally, many studies have demonstrated the importance of insulin signaling in short-term and transient behavioral plasticity as discussed above. Taken together, the proper function of the insulin signaling pathway into old age may be fundamental in preserving behavior and its plasticity throughout the lifespan of an animal. The insulin signaling pathway mediates internal sensation of nutrition, and one of the hallmarks of aging is “actively deregulated nutrient sensing” which relates to behavioral plasticity both in C. elegans and mammals [49,50]. Indeed, it is experimentally observed that ins-1 pathway mutants of age-1/PI3-kinase and daf-2/insulin receptor show extended lifespan as well as enhanced ability to follow their cultivation temperature on a thermal gradient (isothermal tracking) [30]. Compared to wild type, some mutants for INS-1 pathway, DAF-2/insulin receptor and AGE-1/PI3-kinase, were shown to retain the ability to track isotherms into old age [30]. In addition, these mutants have increased ability to learn to associate starvation with a conditioned temperature cue [30].
Long-lived insulin signaling mutants have been previously shown to have more robust defenses against environmental stressors [51], and improved maintenance of the circuit might contribute to the enhanced learning: The increased resistance to stress in mutants might be beneficial to the sustained maintenance of neural functions into old age [30]. However, more research is required to achieve a definitive explanation.
C. elegans is a rather simple organism with a nervous system comprised of only 302 rigidly stereotyped neurons. Still worms can produce complicated behaviors such as associative learning in response to odors, salts, temperature, and other environmental cues. Starvation, as we have outlined in this review, is a powerful driver of such associative learning. A variety of assays on starvation-dependent learning of C. elegans has identified INS-1/insulin signaling pathway as a strong modulator by which nutrient information is encoded in the nervous system. INS-1/insulin acts on neurons that play key roles both in thermo- and chemotaxis circuits, as if to create an integration hub that allows worms to respond adaptively to environmental stimuli. However, the molecular drivers of these adaptive responses vary greatly. In salt chemotaxis experiments, activation of kinases AKT-1 and PDK-1 in salt-sensing neuron ASER is responsible for reversal of salt preference following starvation. Conversely, loss of negative thermotaxis behavior during starvation depends on chemo/thermosensory neuron AWC and changes to downstream circuit dynamics through INS-1/insulin signaling, instead of inducing changes in the primary thermosensory neurons. The fact that a single signaling pathway is able to drive many different experience-dependent cellular changes demonstrates how flexibly INS-1/insulin can be used in the nervous system. This flexibility may be the key in understanding the mechanism that can encode and integrate complicated internal and external information in simple organisms of limited neural computing ability. Similarities between the invertebrate and mammalian insulin signaling pathway should allow C. elegans research to provide insights into the insulin pathways contribution in learning or brain function in higher animals such as mammals. The future research into INS-1/insulin signaling in associative learning is thus vital for understanding universal neural bases of sensory integration in the nervous system.
This review has explored the recent findings on the roles of insulin signaling in starvation-based behavioral plasticity in C. elegans. Insulin is a secreted molecule that allows communication between distant tissues, such as the gut and the nervous system. Here we see that insulin signaling functions in many cell types and has an especially important role in neurons in order to modulate behavior. Insulin signals allow C. elegans to learn associations between various stimuli: Availability of food to odor, salts and temperature. Such ability is important for animals to be able to reconfigure their behavior to maximize foraging efficiency and increase the possibility of survival. In addition to the mechanisms by which insulin signaling modulates chemo- and thermotaxis upon starvation, this pathway is also likely to be important for the preservation and maintenance of neural-circuit functions during aging. Overall, insulin signaling plays an important role in behavioral plasticity in worms as well as other animals, and future research may further elucidate its diverse functions in the nervous system.
This work was supported by JSPS KAKENHI Grant Number 20K15845. We thank M. Shima for critical comments on the manuscript.
Citation: Galatsis KN, Takeishi A (2021) Insulin Signaling Acts Extensively in C. elegans Starvation-Associated Learning and Behavioral Plasticity. J Cell Sci Therapy.S5:315.
Received: 20-Aug-2021 Accepted: 03-Sep-2021 Published: 10-Sep-2021 , DOI: 10.35248/2157-7013.21.s5.315
Copyright: © 2021 Galatsis KN, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.