Journal of Physical Chemistry & Biophysics
Open Access
ISSN: 2161-0398
ISSN: 2161-0398
Review Article - (2015) Volume 5, Issue 2
This article deals with the interaction of the electrolyte ions (saline solution) with the surface of the cell membrane. It is shown that both sides of the bilayer phospholipid membrane surface are negatively charged and the ions, which are in direct contact with the membrane surface, are in relatively deep (as compared to kBT) potential wells, which are localized near the dipole heads of phospholipid membrane. It makes impossible for ions to slip along the membrane surface. This work supports the assumption that the ions located on the excitable axon membrane are tightly bound with it, which underlies the work. Therefore, the electric component of the microwave field, interacting with the ions, transfers energy and momentum directly to the membrane. This interaction leads to forced mechanical vibration of the membrane and, as a result, to a redistribution of transmembrane protein ionic channels. A developed approach can be used for estimations of the surface charges on the outer and inner membrane of the cell. A self-consistent model of the potential in solution is developed, and a stationary charge density on the membrane surface is found. Simple experiments for verifying the correctness of the considered model are proposed.
<Keywords: Lipid membrane; Surface charge; Gouy-Chapman-Stern theory
The question about the distribution of electric charge near the cell membranes is the key for many problems associated with the interaction of cells with external electromagnetic fields [1-6]. For example, if ions are not strongly bound with the membrane and can move freely along it, the electromagnetic field has a weak influence on the membrane. If the ions are firmly bound to the membrane, the electrical component of electromagnetic fields could lead to the membrane deformation [7,8].
The surface charge of the cell membrane has been studied in many experimental works. We will not consider all works and may refer only to [9,10], where the experimental data of the surface charge of cell membranes obtained by different methods are given. A range of results is broad enough: σm=0.3−0.002 C/m2.
The problem of spatial distribution of charge in the vicinity of the biological membranes surface has been considered in many theoretical papers, [1-5]. In all these works, the near-surface potential of the membrane was considered under the Gouy-Chapman theory [11,12] or its later modification by Stern [13], in which the charge on the membrane surfaces is considered to be given. In these theories, the membrane was considered as a continuous dielectric, without taking into account its fine structure, a surface charge was determined on the basis of the electrochemical properties of the dielectric surface [14,15].
In the classical Gouy-Chapman-Stern theory the surface charge ions can freely slide along the surface. Because, it is assumed in this theory, that the interaction of ions with the surface occurs due to electrostatic forces with the induced charges and absorption forces, which depends only on the distance of the ion to the surface. In the case of a phospholipid membrane, the electrolyte ions interact with phospholipid dipoles, which form a mosaic (lattice) structure (Figure 1a) [16]. As a result, the surface charge ions are in a periodic potential Figure 1b, i.e. sliding them along the surface of the membrane is suppressed. This fact is crucial in the theory of interactions of the weak microwave field with the cell membranes, proposed in [17] (see also review [18]), where it was assumed that the longitudinal electric field of the microwave acting on the surface charge causes forced longitudinal vibrations of the membrane, because the surface charge ions are rigidly bonded to the surface and therefore cannot slip.
Figure 1a: A simplified mosaic model of a phospholipid membrane [16]. Spheres represent the dipole heads on the inside and the outside of the membrane.
In our paper, we take into account the fine structure of the bilayer phospholipid membranes [16]. It is shown that the surface of the cell membrane is charged negatively with ions, trapped in the potential wells formed by the dipole heads of the membrane phospholipids. These ions are strongly bound to the membrane surface: the ion binding energy U with the membrane surface is much greater than the thermal energy kBT. That fact allowed the construction of a selfconsistent theory of Gouy-Chapman and Stern theories, and helped to determine the average charge density of sitting on the membrane.
The membrane surface charge theory presented in this paper supports the idea [17] of electromechanical impact of weak microwave electromagnetic fields on the surface density redistribution of transmembrane ion channels.
We emphasize that this article does not discuss the passage of ions through the transmembrane ion channels or pores. We restrict ourselves to considering only the surface processes in the lipid membrane placed in the salt-water solution.
The main ions in the physiological solution ?r?: Na+, K+, ??2+, Mg2+ and ?l-. The total concentration of these: ni ≈ 1.8 × 1026/m3 (300mL/Mole) [19], but for simplicity we will consider only the case when the bilayer phospholipid membrane is immersed into water solution of NaCl.
A model of the phospholipid membranes
The dielectric permittivities of the membrane and water are correspondingly: εm ≈ 2 and εw ≈ 80 [20,21]. It would seem that in order to evaluate the force acting on the ion of charge q near the membrane surface the formula [22] can be applied:
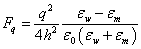 (1)
(1)
Here ε0 is the permittivity of free space, and h is the distance to the interface between dielectrics. However, the paradoxical result follows from the formula (1) that the ion (any sign) cannot get from the dielectric with the higher dielectric permittivity into the dielectric with the lower dielectric permittivity because the force acting on it increases inversely proportional to the square of the distance to the boundary between dielectrics. In other words, the electrolyte ions, regardless of the sign behave like a non-wetting liquid near the surface. This statement contradicts the experimental facts presence of the surface charge on the surfaces of the double phospholipid membrane and theoretical models of Hodgkin and Huxley’s kind, based on the phospholipid membrane permeability to ions.
Usually, in theories of the interaction of ions with the surface of dielectrics, the minimum value of h is selected as the size of the ion, or the Debye radius, only if the insulator’s surface is saturated with ions [15]. However, this does not resolve the problem of phospholipid membranes "impenetrability". In fact, the problem lies that lies within formula (1) is derived in the approximation of continuous medium with the dipoles of the infinitely small size, without taking into account the real structure of the surface layer of the membrane.
It is known that the phospholipid molecules of cell membrane are forming a mosaic (matrix) structure in which dipole heads are directed towards the liquid (positively charged head faces outward membrane) [16]. The average surface area per molecule of the lipid is ≈ 0.5 nm2, the length of the polar head is ~ 0.5–1 nm, the radius of the head is ~ 0.2–0.3 nm, and the distance between the hydrophilic heads of the membrane is in the range of 5-7 nm [19,23]. The dipole moment of the phospholipid head is 18.5-25 D [24] (1D=3.34 × 10−30 Cm), i.e. more than 10 times greater than the dipole moment of water molecules. On the basis of geometrical dimensions of the cell membrane and the size of water molecules, it can be concluded that the free space between the head does not exceed the size of a water molecule (~ 0.2 nm). That is, the membrane, interacting with the ions of surrounding liquid, cannot be considered as a dielectric medium with an infinitesimal dipoles size.
These facts allow the consideration of the following simplified model of the ion interaction with the membrane:
1. The membrane represents a matrix (Figure 2) with a mesh size a×a. The dipoles are located in the nodes of the cells; the dipole charge is q; the distance between the charges (the dipole length), d; the distance between the dipoles along the axis z, l.
2. An ion is a classical particle and cannot approach a dipole at a distance less than the sum of the radii of the head and the size of the ion. It is important that the ion can approach the membrane dipole heads close enough that would be "captured" by the potential well. This is a standard assumption in the theory of the interaction of ions with the surface of the dielectric [15]. Since the dipole moment of water is 10 times less than the dipole moment of the phospholipid head molecule and near the head it cannot be more than one-two water molecules, the interaction between the ions located near the surface of the membrane and the water molecules can be neglected, as compared with the interaction of the ions with the dipoles of the membrane.
3. Once again we want to put an emphasis that ionic permeability membrane is a separate problem and not consider in this work, for us it is important to know how strong ions attached to surface of the membrane.
The potential near the surface of the membrane
The expression for the membrane potential in a cylindrical coordinate system (r,θ,z) at a point above the surface of the membrane (Figure 3):
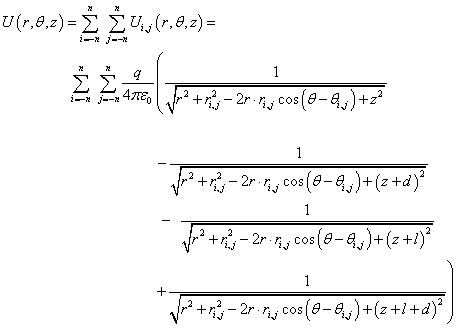 (2)_
(2)_
Here, the radial and angular coordinates ri,j,θi,j, correspond to the position of the dipole in the node with the numbers (i, j), q is the dipole charge, and ε0 is permittivity of free space. The value of n in (2) is chosen so that the potential near the membrane does not depend on the size of the matrix n. The count goes from the node (dipole head) (Figure 3): r=(x2+y2)1/2, θ=arctan (y/x).
We expand the potential U in a Fourier series by θ. Taking into account the rotational symmetry on π/2 rotations for the accepted square matrix of the dipole heads, we obtain:
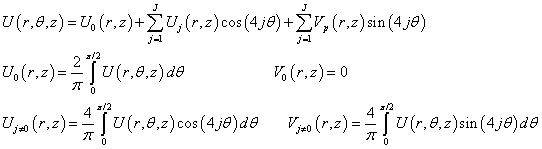 (3)
(3)
In the framework of the model presented above, estimates show that the influence of the dipoles located on the lower side of membrane (Figure 2) on the potential distribution U at z>0 can be neglected. The same is true to the effect of dipoles in the upper side of the membrane on the potential at z<-(l+d). As far as the dependence of U on θ, in the region r
For example, let consider the case, when bilayer phospholipid membrane is immersed in the NaCl solution, at the following set of parameters: a=0.7 nm, d=0.5 nm, l=8 nm, and q=1.6 × 10-19 C 16,19].
Figure 4 shows contour plot of reduced U/kBT for a singly charged ion at T=300K, Figure 5 the dependence U/kBT on the axis z.
It is shown in Figure 5 that the value U/kBT decreases exponentially with the distance from the membrane, wherein a distance z/a=0.5, the ratio U/kBT is about 10. Although it is still a sufficiently deep potential at z/a=1, the influence of the membrane on the ions can be neglected.
An effective potential of the ion across the membrane can be determined. Let the ion be a sphere of radius ri and rh, is the distance from the positive charge of the dipole to the head of the ion (Figure 6).
In this case, the effective reduced ion potential,
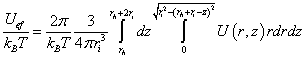 (4)
(4)
The dependence of the effective potential of the ion Cl- in the potential well on the size of the head rh is shown in Figure 6. The chlorine ion radius ri=0.18 nm, and the atomic weight is Mi=35.4.
The typical size of the dipole head rh=0.2 nm (rh/a=0.29) and the ion radius ri=0.18 nm, the reduced potential depth is. Uef/kBT ≈ 10. Thus, the trapped ion is quite firmly bound to the membrane. It should be noted that for electrolytes of a different composition of negative ions, for example in the axon, where the major negative ions are anion groups of macromolecules and phosphates, estimations of the potential will differ from that shown in Figure 7.
Note, that the binding energy of small basic peptides to the membrane containing acidic lipids was determined in the paper [25]. It was assumed in [25] that the membrane is a medium with dielectric constant εm=2, with dipole heads, which form a periodic structure similar to that described above in present article. This membrane is immersed in the electrolyte with ε=80, filling, in particular, the regions between the dipole heads. It was also assumed that the electrolyte ions are absent at a distance of about 2 nm from the dipole heads. Under these assumptions, the potential distribution near the membrane was calculated, based on the Poisson equation, and the binding energy of the peptide molecule to the membrane was determined depending on the distance from the membrane. From our point of view, the physical model considered in [25] has the following drawbacks: first, the distance between the dipole heads in a phospholipid membrane is in order of the size of the water molecule, so it is incorrect to assume that the space between the dipole heads is filled with liquid with dielectric permittivity ε=80. Second, the sizes of the ions K+, Cl- are smaller than water molecules, therefore, it is also incorrect to assume that there are regions near the membrane with the size of ~ 2 nm which has no ions.
Evaluation of the membrane surface charge
For simplicity, we assume the ions as a point charges, but will take into account the finite size of the ion as the minimal distance that the ion can approach to the membrane. As indicated above, this is a standard approach in models of the ions interaction with the dielectric surfaces.
We assume that the space charge on the membrane does not affect its structure, i.e. the dipole heads. Since the field of the membrane (without taking into account the ionic charge on it) at a distance of the order of mesh size is negligibly small, and the distance between the free ions in the solution is larger than the mesh size, we can relate the charge on the membrane surface to the charges in solution by equating the flows at the membrane/liquid boundary (Figure 8).
Let us estimate the flux of ions falling on the membrane. Assuming the Boltzmann distribution of ions outside the membrane:
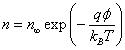 (5)
(5)
Where n∞ is ion density at infinity. Assuming that the ions are in thermal equilibrium with the water molecules, we obtain the following estimate for the ion flux on the surface of the membrane:
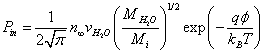 (6)
(6)
Here  is the averaged ion velocities in solution, and
is the averaged ion velocities in solution, and  are the masses of the water molecule and the ion, correspondingly.
are the masses of the water molecule and the ion, correspondingly.
On the other hand, an estimate of the ion flux “evaporating” from the surface of the membrane is as follows. The flow of ions from the surface of the membrane can be considered as a process of evaporation of the liquid with a work function equal to the potential well U. The ion in the well is exposed to random impacts by water molecules, while the ion’s presence time in the potential well τi can be estimated as:
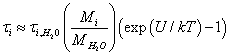 (7)
(7)
Here 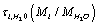 is the characteristic time of energy exchange between the ion and the water molecules. In (7) we have taken into account that at
is the characteristic time of energy exchange between the ion and the water molecules. In (7) we have taken into account that at 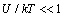 the ions are reflected from the membrane, i.e., the time delay is zero. For estimates
the ions are reflected from the membrane, i.e., the time delay is zero. For estimates 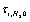 can be assumed equal to the collision time of water molecules in a solution
can be assumed equal to the collision time of water molecules in a solution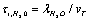 ,
, 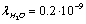 nm is the mean free path of a molecule of water, and vT is the thermal velocity of water molecules. Accordingly, the number of the ions leaving the unit surface of the membrane per unit time is equal to:
nm is the mean free path of a molecule of water, and vT is the thermal velocity of water molecules. Accordingly, the number of the ions leaving the unit surface of the membrane per unit time is equal to:
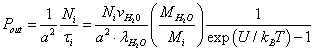 (8)
(8)
Here 1/a2 is the number of potential wells per unit membrane surface; Ni is the relative population of the potential wells with ions. Equating the incident flux of the ions on the membrane to the "evaporating" flow from it, we obtain the relative population of the potential wells:
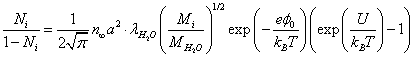 (9)
(9)
In (8) we took into account that the probability of the incident ion being captured for in a potential well is proportional to 1-Ni.
Now we will find the distribution of electric potential in the liquid. Following the Debye approximation for the liquid electrolyte [11]:
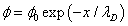 (10)
(10)
Where 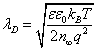 is the Debye length.
is the Debye length.
The field on the membrane surface:
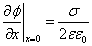 (11)
(11)
Where σ is the surface charge density. In the case when the upper and lower surfaces of the membrane are equally charged σ=2 Niq/a2, we obtain the potential at the membrane surface:
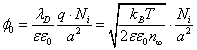 (12)
(12)
Substituting (12) into (9), we obtain the equation for the value of Ni:
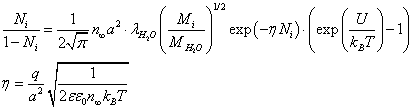 (13)
(13)
For parameter values that are typical for a physiological solution: n∞=0.9×1026/m3, a2=0.5×10-18m2, T=300 K,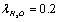 nm, for the chloride ion
nm, for the chloride ion 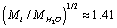 we obtain from (13) η ≈ 13.9, and, therefore,
we obtain from (13) η ≈ 13.9, and, therefore,
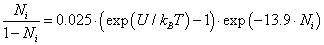 (14)
(14)
The calculated dependence of Ni on the reduced potential 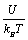 is shown in Figure 9.
is shown in Figure 9.
The maximum surface charge density in Figure 9 corresponds to the case where all of the cells (Figure 1b) are full, i.e. one negative ion is in every cell. It is seen that, even for small values U/kBT, the relative occupancy of the potential wells with charges large enough and the charge density of the charges, which are tightly bounded to the membrane, can reach hundredths of coulomb per square meter. It should be noted that in this estimation we did not consider the interaction between the ions bonded to the membrane, which can certainly play an important role for a more accurate calculation of the occupancy of the potential wells.
Let us estimate the charge of the free ions of one sign in the nonquasineutral region in the vicinity of the membrane surface.
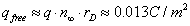 (15)
(15)
For typical values of the potential U/kBT ≈ 5−15, which correspond to the size of the head rh~0.2−0.3 nm (Figure 9), a bounded charge sitting on the membrane surface is much larger than the charge associated with unbounded ions.
In this article, we examined the behavior of the membrane surface with the dipole heads placed in an electrolyte solution. However, despite of this very simple approach, the results are directly related to the processes in biological lipid membranes, such as the membranes of the axon. Because the negative ions inside of the axon differ from those located on the outside (inside the main negative ions are anion groups of macromolecules and phosphates, but chloride ions outside), it is expected that the surface charge density of ions sitting on the membrane is different. Figure 10 shows the potential distribution inside and outside of the membrane where the inner and outer sides of the membrane have the same (A) and different (B) surface charges.
A few words about the experimental verification of the proposed model. As follows from (13), the main parameters that determine the relative occupancy of the membrane with negative ions Ni, are 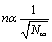 and U/kBT. Accordingly, by changing the density of NaCl in the solution and measuring the surface charge of the membrane, the curves, similar to those shown in Figure 9, can be reproduced. It is shown in [17] that the longitudinal displacements of the membrane in the microwave field is resonant and is linearly dependent on the surface charge density (Figure 10). Accordingly, by changing the salinity of the water, it is possible to determine the surface charge of the membrane and to compare with estimates obtained above (Figure 11).
and U/kBT. Accordingly, by changing the density of NaCl in the solution and measuring the surface charge of the membrane, the curves, similar to those shown in Figure 9, can be reproduced. It is shown in [17] that the longitudinal displacements of the membrane in the microwave field is resonant and is linearly dependent on the surface charge density (Figure 10). Accordingly, by changing the salinity of the water, it is possible to determine the surface charge of the membrane and to compare with estimates obtained above (Figure 11).
The methods of molecular dynamics were used in [24-29] for study of ions Na+ and Cl- penetration in phospholipid membrane. However, in those articles the binding energy of the ions with phospholipid heads was not illustrated. Therefore, it would be interesting to compare the corresponding results of our model and calculations performed by using the molecular dynamics.
It is shown in this paper that:
1. The effective binding energy of the ion with the membrane is of order of several kBT. i.e., the ions are firmly bounded to the surface of the membrane.
2. The value of the potential decays exponentially with distance from the membrane, so that at a distance greater than 0.7 nm the influence on the ions of the electrolyte by the membrane can be neglected.
3. The surface charge ions are in relatively deep potential wells, localized near the dipole heads of the phospholipid membrane, which prevents them from sliding along the surface of the membrane.
4. A qualitative self-consistent theory of the potential distribution near the membrane is considered. It is shown that the density of the bound charges on the membrane can reach hundredths of C/m2.
5. This work supports the assumption that the ions located on the excitable axon membrane are tightly bound with it, which underlies the work [17]. Therefore, the electric component of the microwave field, interacting with the ions, transfers energy and momentum directly to the membrane. This interaction leads to forced mechanical vibration of the membrane and, as a result, to a redistribution of transmembrane protein ionic channels.
It should be noted that the proposed model of the potential distribution near the membrane can be extended to for different types of biological membranes, taking into account their characteristics.