Journal of Aeronautics & Aerospace Engineering
Open Access
ISSN: 2168-9792
ISSN: 2168-9792
Research Article - (2013) Volume 2, Issue 5
It is shown that spark initiated flames of lean hydrogen-air mixtures (8%-15% Ð2) pass through close-meshed aluminum spherical obstacles with the cell size 0.04-0.1 mm2; the flame of 15% Ð2 in air after an obstacle is accelerated; acoustic gas fluctuations occur in the reactor. The flame of 7.5% hydrogen-air mixture does not propagate through the obstacles the flame of 8% natural gas-air mixture is not accelerated after an obstacle; acoustic fluctuations are missing. It is shown that active centers of methane and hydrogen combustion, determining flame propagation, have different chemical nature.
<Keywords: Hydrogen combustion; Chemical nature; Methane
Influence of the obstacles located in different volumes, filled with combustible mixture, on the propagation of Flame Front (FF) is investigated for a long time. These researches are performed to find out both the dependence of combustion regime on the type of obstacles and possibility to influence on combustion regimes by varying of an obstacle shape.
It is known that if the composition of gas mixture is far from concentration limits of ignition the velocity of flame propagation in the presence of obstacles can quickly increase to supersonic values [1,2]. At studying of quickly accelerated flames it is possible to observe DDT (deflagration to detonation transition), however, the velocity of supersonic combustion wave in the presence of obstacles is often below Chapman-Jouquet velocity [3,4]. Therefore from the practical point of view the most prominent aspect in investigation of accelerated flames is caused by problems of engines operation or explosion safety and connected mainly with transition of fast combustion to non-stationary quasi-detonation regimes which destructive influence is more effective than in Chapman-Jouquet regime [5]. Relevance of the researches is connected also with ensuring fire safety in volumes of complicated geometry in particular onboard manned spacecrafts. It is necessary to notice that influence of obstacles, according to [1], can be expressed in the double way. One can observe either the occurrence of a detonation wave due to reflections of shock waves or quenching of a detonation wave as a result of heat losses.
It is possible to carry the aforesaid to an initial stage of flame acceleration, namely to the moment when the laminar flame meets an obstacle of net shape; that is the subject of the present research. This interaction causes the development of flame instability, promoting its acceleration [6]. On the other hand, the contact of FF with reactor surface leads to increase of the contribution of heterogeneous reactions, in particular chain termination [7]. This should promote flame suppression. This ambiguous mechanism of obstacles action underlies that physical means of detonation suppression (nets, nozzles etc.) [8] are not always effective.
The influence of obstacles (nets and perforated spheres with the minimum cell size 2×2 mm and aperture diameter of 4 mm accordingly), located in combustible gas, on the visible velocity of combustion of stoichiometric hydrogen-air and hydrogen -oxygen mixtures was investigated in [2]. Acceleration of FF in 1.5-2.5 times was always observed after obstacles. DDT was observed for hydrogenoxygen mixtures, depending on energy of initiation [2].
However, the data on interaction of flames of lean hydrogenair mixtures with penetrable obstacles are practically absent in the literature, though such experiments are of interest for an establishment of influence of opposite factors: flame accelerating (instability development) and flame suppression (chain termination on obstacle surface).
The work is aimed to investigation of flame propagation dynamics of lean hydrogen-air mixtures and stoichiometric one of natural gas with air in net sphere, FF propagation through a net sphere and the further propagation of FF outside the net sphere.
Experiments were performed with lean mixtures of hydrogen (7.5- 15% H2)-air and stoichiometric mixture of Natural Gas (NG) with air at initial atmospheric pressure and 298К in a horizontally located stainless steel cylindrical reactor of 15 cm in length and 13 cm in diameter. The reactor was supplied by an optical quartz window on one of its butt-ends (Figure 1). Electrodes of spark ignition (6) were located in the reactor centre; the distance between them was 0.5 mm. On these partially isolated electrodes (6) the net sphere (5) was fixed, with the cut out grooves for the electrodes. The net sphere consisted of two hemispheres fastened by a spring (7). Thus the volume included in net sphere, and the external reactor volume contacted only through net cells. The net was made from aluminum wire. Net spheres of 3 cm in diameter (diameter of wire 0.2 mm, cell size 0.04 mm2), d=4 cm (diameter of wire 0.25 mm, cell size 0.08 mm2), d=6 cm (diameter of wire 0.3 mm, cell size 0.1 mm2) were used. As it is known, the aluminum surface is always covered with its oxide. Hence, the net surface consisted of aluminum oxide Al2O3 which effectively terminates active centers of combustion (reaction chains) [6].
Experiments were performed as following. First the reactor was filled with CCl4 (if needed, for better visualization of H2-air flame). Notice that the additive up to 4% ССl4 for the given mixtures is inert [9]. Then the reactor was filled with H2, or Natural Gas (NG), and air was added to 1 atm. The mixture was maintained for 15 min for completeness of mixing and then the spark initiation was performed (discharge energy made up 1.5 J). Recording of dynamics of ignition and FF propagation was carried out through an optical window with color high-speed digital camera Casio Exilim F1 Pro (frames frequency–60-1200 s-1). The video file was stored in computer memory and its time-lapse processing was performed.
The pressure change of in the course of combustion was recorded by means of a piezoelectric gage. Before each experiment the reactor was pumped out to 10-2 Torr. The degree of expansion of combustion products εT was determined as follows [6] (Рb is maximum pressure developed in the course of combustion):
 (1)
(1)
The normal velocity Un of FF was calculated from the equation [6]:
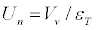 (2)
(2)
In equations (1), (2) P0–initial pressure, γ=1.4-the ratio of specific heats, Vv-visible flame velocity.
In all experiments with hydrogen flames the flame initiated in a net sphere passed through net cells (except the mixture 4% ССl4 +7.5% Н2+air). It means that interaction of a rather slowly propagating flame (normal velocity of FF in 15% Н2 with air (≈ 50 cm/s) is ~ 6 times less than in the stoichiometric flame; the normal velocity of FF in 10% Н2 with air (≈ 20 cm/s) is ~ 15 times less than in the stoichiometric flame [9]) with a surface providing effective chain termination (Al2O3), doesn’t lead to flame suppression. Thus, the influence of heterogeneous chain termination of H atoms on flame propagation isn’t enough for flame quenching under our conditions.
It has been established that the flame in 4% ССl4+7.5% Н2-air mixture doesn’t pass through net spheres; however in the sphere separate flame cells caused by thermal diffusion [9] are observed after initiation. If there is no net sphere in the reactor, cellular flame rises upwards as in [10]. Thus, under our conditions there is critical concentration of H2 at which the flame doesn’t pass through a net sphere. It is in agreement with calculations [11] where it is shown that the influence of chain termination on flame propagation should be observed in the immediate vicinity to the lower concentration limit of flame propagation (which for hydrogen-air mixtures make up ~ 5% H2 [9]).
The sequences of video images of FF propagation illuminated with 4% ССl4, in mixtures of 10% Н2 and 15% Н2 with air correspondingly, illustrating the influence of a net sphere on dynamics of flame propagation are presented in Figures 2 and 3. As is seen after propagation through the net sphere FF is considerably disturbed in comparison with flame propagation without a net sphere. As is seen in Figure 2 FF consists of cells (of the thermal diffusion nature, see above) which become smaller with increase in H2 concentration in agreement with [9,11]; thus in the course of FF propagation long-wave disturbances occur.
Figure 2: High-speed filming of FF propagation illuminated with 4% ССl4, in 10%Н2 + 86% air at atmospheric pressure. The figure on a frame corresponds to the frame number after discharge. The first frame corresponds to spark emergence in gas. Speed of filming 60 frames/c. a)–no obstacle; b)–in the presence of the net sphere d=6 cm
Figure 3: High-speed filming of FF propagation illuminated with 4% ССl4, in 15%Н2 + 81% air at initial atmospheric pressure. The figure on a frame corresponds to the frame number after discharge. Speed of filming 600 frames/c
a)–no obstacle; b)–in the presence of the net sphere d=4 cm c-Dynamics of increase in radius of FF without (1) and in the presence (2) of net sphere d=4 cm.
Dynamics of increase in a FF radius in absence and in the presence of a net sphere is shown in Figure 3c. As is seen FF in the vicinity of the net sphere is slowed down, however after propagation through the obstacle FF is considerably accelerated in agreement with [2]. Then at the reactor wall FF is slowed down again due to the change in conditions of expansion of combustion products [6,9,12,13]. One can see the occurrence of bright streams of hot gas from the volume inside of the net sphere out of it which arise after FF reaches the reactor walls (shots 17-19 Figure 3b). As it was shown specially, these streams are due to small glowing Al2O3 particles carried away by a gas stream. In accordance with contemporary representations of hydrogen combustion secondary exothermic reactions are missing in the process [9]. In addition the presence of a net sphere should lead to faster cooling of gas inside it. Therefore the gas stream should be directed into the net sphere. However the gas stream is directed outside it. The determination of the nature of this phenomenon demands more detailed research.
It has been established that in the presence of net spheres flame propagation in mixtures of both 10% Н2 and 15% Н2 in air is accompanied by a characteristic sharp sound, i.e. acoustic fluctuations of gas occur. Notice that flame propagation in the mixture of 10% Н2 with air without a net sphere isn’t accompanied by sound effect. The dependencies of change in total pressure on time for flame propagation in specified mixtures in the presence of the net sphere are shown in Figures 4 and 5. As is seen in Figure 4b at the time ~ 365 ms acoustic fluctuations occur in the presence of the net sphere during the combustion of 10% Н2-air mixture, and the time of pressure raise becomes shorter in comparison with the process without the net sphere in accordance with [2].
As is seen in Figure 5a during combustion of 15% Н2- air mixture without the net sphere acoustic fluctuations occur after pressure maximum in the time interval 110 ÷ 125 ms. In the presence of the net sphere of d=3 cm (Figure 5b) acoustic fluctuations arise considerably before pressure maximum at the time ~ 95 ms, i.e. the presence of the obstacle leads to faster development of a flame instability and provides intensification of combustion. Notice that in a spherical bomb of larger diameter (38.4 cm) (Figure 3 [14], curve ф□=0.4) acoustic fluctuations arise before the pressure maximum. It is necessary to pay attention to the fact that the larger diameter of the net sphere is, the later acoustic fluctuations arise. As is seen in Figure 5c acoustic fluctuations are raised after the pressure maximum at the time ~ 115 ms. It means that the presence of a net obstacle leads to development of instabilities on FF and to occurrence of acoustic fluctuations.
Figure 5: Oscillograms of pressure change in ignition of gas mixtures.
4%CCl4 +10% H2 in air; a– without net sphere; b– in the presence of net sphere d=3 cm; c–in the presence of a grid d=4 cm; 1–a signal at the initiated ignition, 2–a comparison signal, large points-positions of the cursor of the oscilloscope which fix time interval, shown below each oscillogram; 3–the stretched time interval.
In the following series of experiments it has been shown that the combustion of stoichiometric NG-air mixtures for all net spheres used in the present work completely covers the reactor volume. However, unlike combustion of hydrogen-air mixtures, at inner surface of the net sphere FF actually stops and its luminescence vanishes (Figure 5).
It means that the mechanism of penetration of the flame of NGair mixture through the net obstacle differs from the mechanism for hydrogen-air mixtures. The sequence of video images of flame propagation in NG-air mixture through the net obstacle is shown in Figure 6а, the dynamics of increase in the FF radius in the presence of a net sphere is shown in Figure 6b. As is seen from Figure 6a at approach of FF to inner surface of the net sphere the flame front practically disappears (shot 20). At combustion out of the obstacle FF isn’t accelerated but propagates with almost constant velocity. Therefore the excitation of acoustic fluctuations at the expense of flame acceleration in combustion of this mixture does not take place and is not experimentally recorded. The estimation of normal velocity of flame propagation out of the obstacle using equations (1) and (2) gives ~ 27 cm/s; this value is close to the normal velocity of a spherical flame for this mixture composition (35 cm/s [9]). One can assume that attenuation of NG -air flame is connected with intensive heterogeneous destruction of active intermediate products of combustion on the net surface. However stable intermediate products of combustion (for example, hydroperoxides) diffusing through net cells again initiate flame propagation outside of the net sphere. The absence of a sound effect at combustion of the mixture testifies in favor of this assumption, i.e. presence of a net obstacle doesn’t lead to FF instability and to occurrence of acoustic fluctuations.
Figure 6: a- High-speed filming of FF propagation in stoichiometric mixture of NG with air at atmospheric pressure in the presence of net sphere d=4 cm The figure on a frame corresponds to frame number after discharge.The first frame corresponds to spark emergence in gas. Speed of filming 600 frames/s b–Dynamics of increase in radius of FF in the presence of net sphere.
Let’s specify that normal velocities of FF of mixtures 10% Н2 and 15% Н2 with air make up 21 cm/s and 45 cm/s correspondingly in agreement with [10] in which the data of several groups of authors on determination of Un and a curve of its average values is presented. Notice that the normal velocity of flame propagation in stoichiometric NG-air mixture makes up ~35 sm/s, i.e. the values of Un for three mixes under investigation are close to each other.
However the partial heat of combustion of NG is considerably higher than that of hydrogen [9]. Therefore the disappearance of NGair flame at the net obstacle has no explanation on the basis of the only thermal theory 6. It means that in the work the direct evidence is obtained that the active centers of combustion of methane and hydrogen, determining flame propagation, have the different chemical nature [15,16]. The reason of close-to-zero velocity value of NG-air flame in the vicinity of net obstacle is due to the fact that light hydrogen atoms easily penetrate through the net obstacle, but chain carriers of methane combustion, on the contrary, are effectively terminated on Al2O3.
It is shown that the flames of lean hydrogen air mixtures (8%-15% Н2) can propagate through aluminum net spheres (cell size 0.04-0.1 mm2); the flame of 15% Н2 in air after obstacle is accelerated; acoustic gas fluctuations occur in the reactor. The less diameter of net sphere is the earlier acoustic fluctuations occur. On the contrary the flame of 8% natural gas-air mixture passes through obstacles relatively slow; after the obstacle flame velocity remains constant; acoustic fluctuations aren’t experimentally observed. The conclusion is made that active centers of methane and hydrogen combustion, determining flame propagation, have different chemical nature.