Journal of Plant Biochemistry & Physiology
Open Access
ISSN: 2329-9029
ISSN: 2329-9029
Review Article - (2022)Volume 10, Issue 8
Plants respond and adapt to their environment for survival and reproduction. Plant growth, development and fitness are important factors affecting crop production and food security. Plants have evolved inducible defenses in response to biotic and abiotic stresses regulated by temperature, light and circadian rhythm. Activation of plant immune signals against particular pathogen infection is a securely controlled mechanism wherein defense responses are specifically turned on upon recognition of pathogen. As a result, adverse effects on plant growth and development can be controlled and minimized. It remains elusive how interactions between plants and pathogens operate the immune signaling responses in relation to environmental fluctuations. This paper summarizes recent studies demonstrating the interactions between plant innate immunity, temperature, light and circadian rhythm. In addition, our review identifies further research questions and implications for experimental design and analysis to elucidate the complex relationship necessary to explain the interaction between plant immunity temperature, light and circadian rhythm.
Plant immunity; Circadian clock; PAMP-triggered immunity; Effector-triggered immunity; Plant signaling pathway.
PAMPs: Pathogen Associated Molecular Pattern Molecules; PTI: PAMP-triggered Immunity; ETI: Effector Triggered Immunity; ICS1: Isochorismate Synthase 1; SA: Salicylic Acid; NPR1: Nonexpresser of Pr Genes 1; BTH: Benzothiadiazole; TGA: TGA transcription factors; PR1: Pathogenesis Related Gene 1; UBC13: Ubiquitin Conjugating Enzyme 13; ZRKs: ZED1 Related Kinases; ZAR1: Zygotic Arrest 1; SNC1: Suppressor of NPR1-1; SA: Salicylic Acid; ELF3: Evening Expressed Early Flowering 3; ZTL4: Zeitlupe-4; TOC1: Timing of Cab Expression 1; CCA1: Circadian Clock Associated 1; LHY: Late Elongated Hypocotyl; GRP7: Glycine-rich RNA-binding Protein 7; CCGs: Clock Controlled Genes; PIF4: Phytochrome Interacting Factor 4; FHY3: Far-red elongated Hypocotyl 3; FAR1: Far-red Impaired Response 1; SIZ1: SUMO e3 ligase which is an orthologous of the mammalian; PIAS: Protein Inhibitor of Activated STAT and yeast SIZ (SAP/Miz) proteins; COP1: Constitutive Photomorphogenic 1; HY5: Elongated Hypocotyl 5; SNC1: Suppressor of NPR1-1 Constitutive 1
Plant diseases are one of the most important causes of crop yield reduction worldwide posing a big challenge to human food production and demand. Food security and biodiversity are affected by climate change and unprecedented fluctuations in temperatures [1]. Both biotic and abiotic factors play an important part in regulating plant–pathogen interactions and it was known that the epidemic diseases are more likely to increase when environmental conditions are detrimental for plants [2,3]. The rise of ambient temperature optimizes plant growth and development and compromise plant immune responses resulting in increased susceptibility against pathogens [4-6]. Suppression of plant defense responses at higher temperature mediates the activation of genes between growth and defense [7]. In-depth knowledge on how specific environmental factors affect the interaction between plants and pathogens can further identify effective disease control strategies to increase crop yield and develop highly resistant crops under increasingly unpredictable climatic conditions.
The success of plant development, survival and reproduction is influenced by plant’s response and adaptation to their immediate environment. Plants utilize energy and resources to enhance its biotic and abiotic defenses and maintain growth and development. Moreover, plant immunity against harmful pathogens undergoes a systematic activation of defense signaling cascades upon direct recognition of pathogen. The specialized signaling processes found in plants regulate immune responses upon perception of incoming pathogen attack. Plant pathogens are equipped with the ability to compromise plant immune signaling and have the capacity to evolve. With this, plant immune signaling activates a higher level of resilience to protect itself against pathogens. Unnecessary immune induction costs plant fitness which can cause damage to both plants and pathogens [8]. Plant immune signaling network also possess the ability to quantitatively tune its output according to the reliability of pathogen attack cues in order for plants to use correct molecular cues in detecting pathogens from non- pathogens [9].
Plant defense is composed of various immune mechanisms called, PAMP Triggered Immunity (PTI) and Effector Triggered Immunity (ETI) [10,11]. Pathogen Associated Molecular Patterns (PAMPs) are perceived by Receptor Like Kinases (RLK) on the cell membrane to activate PTI. Pattern Recognition Receptors (PRRs) trigger innate immune responses in plants. One such PRR is the flagellin receptor FLS2 in Arabidopsis. Flagellin and elongation factor Tu in bacteria are PRRs perceived by Leucine-rich Repeat RLK (LRR-RLK), Flagellin Sensitive 2 (FLS2) and Ef-Tu Receptor (EFR) in Arabidopsis, respectively [12] (Figure 1).
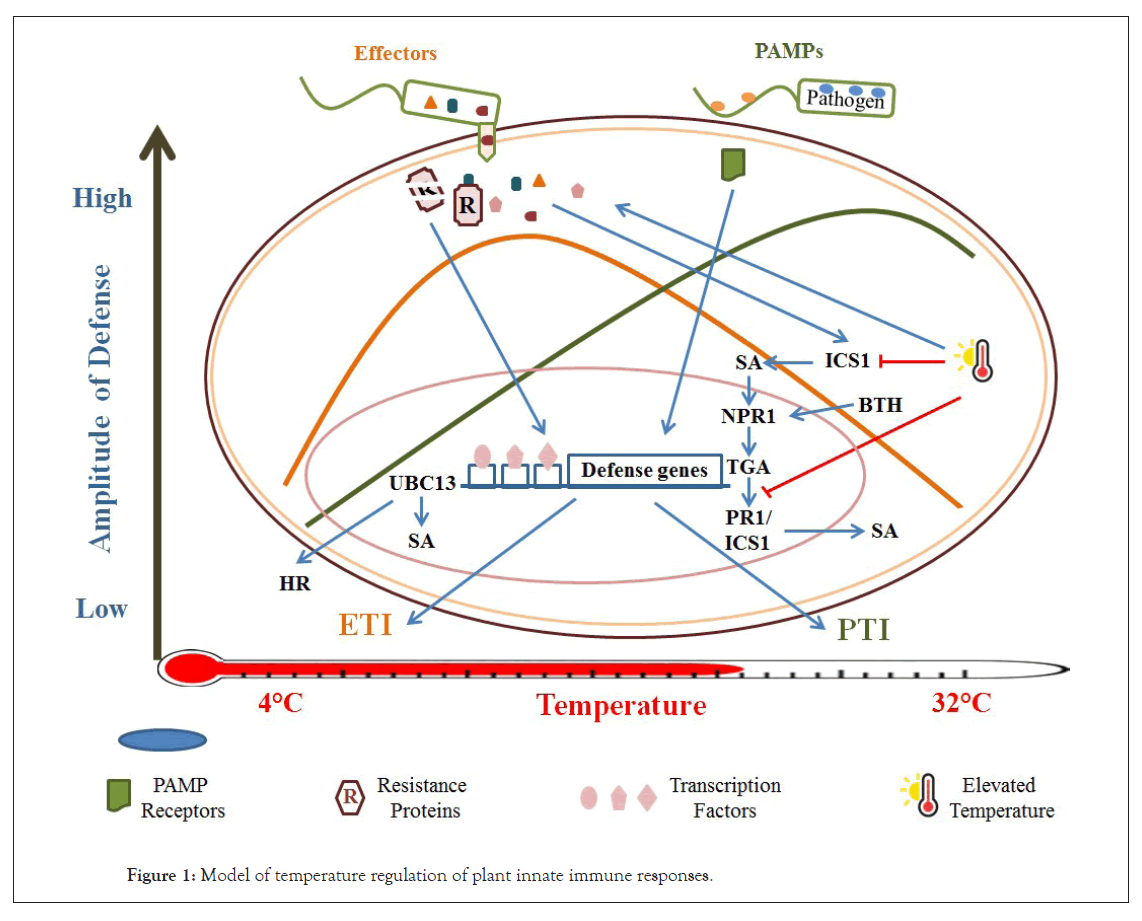
Figure 1: Model of temperature regulation of plant innate immune responses.
a) Bacteria secrete various amounts of virulence effectors to promote Effector-triggered Susceptibility (ETS) which activates Effector-triggered Immunity (ETI) signaling at low ambient temperature. Pathogens produce increased amount of PAMPs which lead to activation of PTI signaling at elevated temperatures.
b) Pathogen induces SA biosynthesis at elevated temperature by blocking ICS1 enzyme and increased translocation of bacterial effector proteins to promote virulence. SA signaling induced by the SA synthetic analogue, BTH, is also affected wherein PR1/ ICS1 are suppressed at elevated temperature. BTH-mediated resistance against pathogen is conferred at elevated temperature in NPR1-dependent manner.
c) UBC13 has a role in plant response to low-temperature stress. UBC13 induces the expression of several cold- responsive genes promoting hypersensitivity to low-temperature stress. Furthermore, UBC13 regulates programmed Hypersensitive Response (HR) in response to low temperature and pathogen.
Perception of PAMPs initiate downstream signaling of immune response networks which lead to transcriptional reprogramming of defense genes, enhanced callose deposition in the cell wall and increased Reactive Oxygen Species (ROS) [12,13]. Adapted pathogens are able to repress PTI through production of virulence effectors into the plant cell which disrupt PTI signaling and maximize cellular proliferation [10]. The PTI-compromised state is known as Effector-triggered Susceptibility (ETS).
Plants evolved another way to detect pathogen attack by directly or indirectly recognizing some pathogen effectors by cognate resistance (R) proteins [11,14]. Bacteria deliver effector proteins into plant cells via Type III Secretion System (T3SS) to promote pathogen survival and reproduction. Effectors target plant cell components to suppress immune responses and physiology. Effectors of the Gram-negative bacterial pathogen Pst, AvrRpt2 and AvrRpm1, are recognized by R proteins RPS2 (Resistance to P. syringae 2) and RPM1 (Resistance to Pseudomonas syringae pv. maculicola 1), respectively [15-17]. To eliminate pathogenic bacteria, plants developed intracellular Nucleotide-binding domain Leucine-rich Repeat (NLR) proteins to recognize effectors and lead to activation of ETI. ETI is associated with robust immune responses which includes local programmed cell death in plant cells at the infection site known as Hypersensitive Response (HR), production of salicylic acid, and induction of Systemic Acquired Resistance (SAR) [18-21]. In addition, RNA silencing recognizes aberrant RNAs derived from viruses and it operates to defend plants against viruses through systematic degradation of RNA products [22,23] (Figure 2).
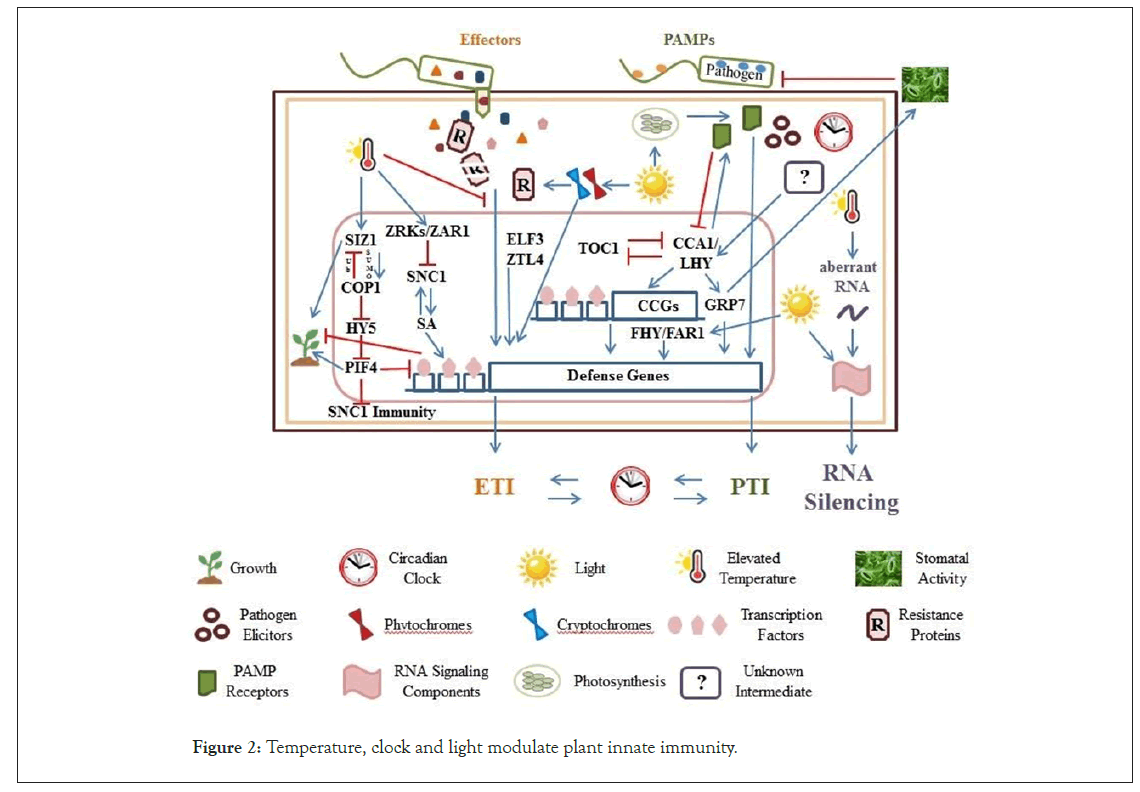
Figure 2: Temperature, clock and light modulate plant innate immunity.
Plant immunity is divided into 3 defense mechanisms namely, PTI, ETI and RNA silencing which are triggered by PAMPs, effectors, and aberrant RNAs from viruses, respectively. Temperature, clock and light are abiotic factors which play an important role in plant immunity.
a) Expression of defense genes including R genes is regulated by temperature. Oftentimes, elevated temperatures suppress ETI and enhance activities of RNA silencing component machineries. The expression of ZED1 and some ZRKs are induced at ambient high temperature. ZRKs act cooperatively to inhibit the salicylic acid-dependent defense response pathway by suppressing SNC1 transcription in the absence of pathogens. The SNC1 autoimmunity is suppressed by PIF4 at elevated temperature. The SIZ1 sumoylation (SUMO) of COP1 activates the intrinsic Ubiquitin (Ub) E3 ligase activity of COP1 resulting to degradation of COP1 substrates such as HY5 and SIZ1.
b) Photosynthesis and photoreceptor signaling are components of light which affect defense signaling. Light quality and quantity are integrated into immune responses through different photoreceptors. Phytochromes absorb the red and farred region of the spectrum while crypto chromes absorb blue light. Photoreceptors and specific interacting proteins directly modulate protein stability of R proteins.
c) Circadian clock regulates expression of defense response genes in PTI and ETI. Pathogen elicitors provide the input to the circadian clock via an unknown intermediate. Therefore, circadian clock modulates the expression of various defenserelated Circadian-Controlled Genes (CCGs) via putative binding of clock components such as CCA1/LHY to the promoter region of CCGs. ELF3 and ZTL-4 are clock components which regulate plant immunity. Circadian clock also activates both stomatadependent and independent defense pathways to restrict pathogen growth in Arabidopsis. CCA1 and LHY act through GRP7 as a direct downstream target to regulate stomatal aperture and activate defense in a stomata-dependent pathway. Furthermore, PTI induced by PAMP receptor flg22 to regulate clock activity, thus, clock-defense crosstalk involves flg22-mediated signaling. CCA1/LHY represses the expression of TOC1.
d) PIF4 serves as a negative regulator of plant immunity and its activation affects the balance between growth and defense.
In this paper, we describe the molecular mechanisms to explain how environmental factors and temperature fluctuations affect plant immune responses. Research have shown that temperature influences plant resistance against disease and in this paper, we describe the molecular mechanisms on how temperature-initiated signals affect plant immune signaling pathways. Defense responses are influenced by abiotic factors namely, temperature, circadian rhythm and light which means that different environmental signals communicate with defense responses to stabilize growth and immunity. At present, the ambiguous junction between abiotic and biotic responses remains as a big scientific controversy. Here, we outline the recent discoveries on the molecular mechanisms related to the interlocking relationships between temperature, light and immune responses in plants.
Plant immunity and temperature
Climatic and weather conditions carry critical roles in establishing results of plant-pathogen interactions. It is known that the increase of epidemic diseases occur when environmental factors are optimum for the pathogens to survive and multiply. Breeding efforts to increase crop yield reduce the genetic diversity which increases susceptibility to diseases and caused negative impact on the resilience of plant immunity under conflicting environmental conditions.
Temperature is one of the environmental factors which influence the interaction between plants and pathogens. It was found that the decrease in temperature inhibits interaction between plants and pathogen and disrupts increase in plant growth. Previous studies reported that temperature dependent autoimmunity caused by allelic discrepancies in hybrids of Arabidopsis thaliana accessions revealed that plants maintain ‘poise’ between immune response and robustness in natural populations [5]. The Arabidopsis chilling sensitive 3 (chs3-1) mutant showed deficient growth and chlorosis when grown at 16°C or when shifted from 22 to 4°C. chs3-1 plants also showed constitutively induced defense responses at 16°C, which were increased at 22°C [24]. It was found that tomato spotted wilt virus resistance in pepper conferred by the TSW gene is less stable at continuous 32°C temperature and continuous high temperatures for nine days resulted to systemic spread and necrotic symptoms in plants that are resistant at lower temperature (22°C). Continuous increase in temperature destabilize plant resistance in young plants, however, older infiltrated plants develop less systemic symptoms [25]. In addition, increased temperature activates local viral amplification and cell-to-cell movement of Melon Necrotic Spot Virus (MNSV) but suppress expression of systemic symptoms. The effect of 15, 20, and 25°C on the local and systemic accumulation of MNSV revealed that the systemic symptoms decreases as temperature rises from 20 to 25°C and increases as temperature falls from 25 to 20°C [26]. These findings showed distinctive links between temperature responses and defense responses.
Studies showed that Indole-3-Butyric Acid Response 5 (IBR5) encodes a putative dual- specificity protein phosphatase. IBR5 mutation restricts the accumulation of Chilling Sensitive 3 (CHS3) protein at chilling temperatures. The chs3 defense phenotypes were interdependently suppressed by mutations in Heat Shock Protein 90 (HSP90) and IBR5. IBR5 induce holdase activity and physically associates with Suppressor of the G2 allele of skp1 (SGT1b), CHS3 and HSP90 to create a complex which conserves CHS3 [27]. Research findings showed that mutants capable of activating defense responses and increase accumulation of SA such as (Sensitive to Low Humidity 1 (SLH1)) slh1, (Constitutive Expressor of PR Genes 1 (CPR1)) cpr1-1 and cpr5-2 mutants showed increased resistance to freezing.
It was reported that Phyto chrome Interacting Factor 4 (PIF4) positively enhances growth and development but negatively regulates plant immunity. Regulation of PIF4 function influences temperature sensitivity of defense and disruption of PIF4-mediated growth resulted in temperature-resilient disease resistance in plants [28]. Moreover, Arabidopsis mutant zed1-D showed high temperature dependent growth retardation. A dominant mutation in hopz-eti-deficient 1 (ZED1) is responsible for a high temperature-dependent autoimmunity and growth suppression in zed1-D. The autoimmune phenotype in zed1-D is dependent on the Hopz-Activated Resistance 1 (ZAR1). ZED1 and other ZED1-related Kinases (ZRKs) are activated by high temperature and function cooperatively to suppress the immune response by regulating the transcription of Suppressor of NPR1-1 Constitutive 1 (SNC1) in the absence of pathogens [29]. Furthermore, increased temperature significantly enhances susceptibility of Arabidopsis to Pseudomonas syringae pv. Tomato DC3000 (Pst) independently of the (Phytochrome B (PHYB)) phyB/PIF thermosensing pathway [9].
Studies showed that increased ambient temperature inhibits plant immunity as well as auto- immunity. SIZ1 is a SUMO E3 ligase which is an ortholog of the mammalian PIAS (Protein Inhibitor of Activated STAT) and yeast SIZ (SAP/Miz) proteins. The Arabidopsis sumoylation mutant siz1 showed SNC1-dependent auto-immunity at 22°C and 28°C, which was dependent on Enhanced Disease Susceptibility 1 (EDS1) at both temperatures. It was found also that SIZ1 inhibit the SNC1-dependent resistance response in both ambient and high temperatures. In addition, SIZ1 enhances the dark and high temperature growth response via Constitutive Photomorphogenic 1 (COP1) and upstream of gene regulation by Brassinazole-resistant 1 (BRZ1) and PIF4. COP1 E3 ligase activity modulates increase of PIF4 protein level however COP1 activity is regulated by SIZ1-dependent sumoylation. Previous reports revealed that COP1 is important to regulate the high temperature signal and SIZ1 enhances both dark signal and high temperature. With this, SIZ1 plays role in both immunity and growth at elevated ambient temperature wherein it has a positive role in plant growth; however, it functions as a negative regulator of the SNC1-dependent immune response [30].
Increase in temperature results to down-regulation of immunity and sensitivity of plants to biotrophic pathogens [31]. N. tabacum plants showed severe symptoms at early stage after infiltration of Cucumber Mosaic Virus (CMV) at 28°C associated with higher viral replication level. On the other hand, 18°C effectively delayed the replication of CMV compared with higher temperatures [32]. Leptosphaeria maculans causes blackleg disease which is considered as one of the major threats to canola farming in western canada. Results showed that three QR cultivars have severe lower blackleg relative to westar suggesting that the QR traits are helpful for blackleg regulation in western canada, especially under high temperature growth conditions when plants are young [33]. Furthermore, resistance conferred by the Mi-1 gene from Solanum peruvianum was effective and widely used for managing rootknot nematode (Meloidogyne spp.) yield loss in tomato (Solanum lycopersicum). However, the resistance was suppressed at soil temperatures above 28°C. Most of the LA2157 plants inoculated with the TRV-Mi construct, Mi-9-meditated heat-stable root-knot nematode resistance was compromised at 32°C, indicating that the heat-stable resistance is regulated by a homolog of Mi-1 [34]. Moreover, the pepper cultivar cv. Sy-2 (Capsicum chinense) showed growth defects at temperatures below 24°C. The accumulation of high level of salicylic acid in cv. Sy-2 grown at 20°C suggested that the immune response is induced in the absence of pathogens. Defense response was also induced in cv. Sy-2 below 24°C and cv. Sy-2 also revealed enhanced resistance to X. campestris pv. vesicatoria [35].
The family of 21 genes found in Arabidopsis genome encodes the Toll Interleukin Receptor (TIR); Nucleotide Binding site (NB)- type (TN) proteins. Studies showed that a mutation in the TN gene Chilling Sensitive 1 (CHS1) activates defense responses at low temperatures. It was found that CHS1 interacted with the NB and LRR domains of SOC3 which is a TIR-NB-leucine-rich repeat (TNL) protein. However, mutated chs1 interacted with the TIR, NB and LRR domains of SOC3 both in vitro and in vivo [36]. Changes in Hsp70 gene expression were observed and protein level in three Solanum spp. genotypes in response to high and low temperatures upon inoculation of powdery mildew. Exposure to high temperature increased Hsp70 content in all three genotypes of Solanum spp. [37-122].
The changes in temperature are associated with alterations in different abiotic factors such as humidity and light. Arabidopsis mutants or incompatible hybrids of natural accessions that have constitutively induced immunity in the absence of pathogens displayed severe growth defects. However, auto-activated resistance phenotypes are actively regulated by both humidity and temperature. Therefore, changes in weather and climatic conditions affect the trade-off between immunity and growth in plants (Table 1).
| Mutant/hybrid | Gene | Temperature effects | References |
|---|---|---|---|
| chs3-1 | Chilling Sensitive 3 (CHS3) | dwarfism (16°C) | [24] |
| normal size (22oC) | |||
| bon1 | Bonzai1 (BON1) copine- like protein | dwarfism (22°C) | [6],[48] |
| normal size (28°C) | |||
| bap1, mkp1, srfr1 cpr1/cpr30 | Bon Association Protein 1 (BAP1) | dwarfism (22°C) | [50] |
| Mitogen-activated Protein Kinase Phosphatase 1 (MKP1) | normal size (28°C) | ||
| Suppressor of RPS4- RLD 1 | |||
| Constitutive Expressor of PR genes 1 (CPR1/CPR30) | |||
| zed1‐D | HOPZ‐ETI‐Deficient 1 (ZED1) | dwarfism (24-28°C) | [29] |
| normal size (18-22°C) | |||
| snc1 | Suppressor of NPR1-1 Constitutive 1 (SNC1) | dwarfism (22°C) | [76] |
| normal size (28°C) | |||
| bon1,bon3 | Bonzai 1 (BON1) Bonzai 3 (BON3) LCDs | dead (22°C) | [122] |
| normal size (28°C) | |||
| mekk1 | Mapk/Erk Kinase Kinase 1 (MEKK1) | dwarfism (22°C) | [70] |
| normal size (28°C) | |||
| ssi4 | Suppressor of npr1-5 based Salicylic Acid Insensitivity (SSI4) | Relative humidity (RH): dwarfism (60% RH) | [113], [12] |
| normal size (95% RH) | |||
| mkk1, mkk2, mekk1, mpk4 | MEKK1 (Mapk/Erk Kinase Kinase 1) /SUMM2 | dwarfism (22°C) | [52] |
| normal size (28°C) | |||
| chs2 | Disease resistance protein RPP4 | dwarfism (16-18°C) | [56] |
| normal size (22°C) | |||
| Ler/Kas-2 Ler/Kond | RPP1-like TIR-NB-LRR locus Ler Strubbelig- Receptor Family 3 (SRF3) Kas-2 or Kond | dwarfism (14°C) | [5] |
| normal size (22°C) | |||
| Uk-1/Uk-3 | RPP1-like TIR-NB-LRR locus Uk-1 SSI4 Uk-3 | dwarfism (16°C) | [71] |
| normal size (23°C) |
Table 1: Examples of immune responses that are affected by temperature related to defense responses in autoimmune plants.
| Pathogens | R-gene / defense mechanism | Temperature effects | References |
|---|---|---|---|
| Pseudomonas syringae | RPS4 | Defense suppressed at 28°C | [49] [54] |
| Root knot nematode | Mi-9 | Stable at 28°C | [34] |
| Powdery mildew | RPW8 | Defense suppressed at 30°C | [58] |
| Tobacco mosaic virus | N | Defense suppressed at 28°C | [42] |
| Root knot nematode | Mi-1 | Defense suppressed at 30°C | [57] |
| Rice blast fungus | Pib | Induced by high temperatures | [103] |
| Wheat rust fungus | Yr6 | Induced by high temperatures | [23] |
| Cladosporium fulvum | Cf-4, Cf-9 | Defense suppressed at 33°C | [64] |
Table 2: Examples of immune responses that are affected by temperature related to plant defense responses against pathogens.
The expression of virulence genes in most pathogens is a highly regulated phenomenon affected by variety of parameters namely; iron levels, osmolarity, growth phase, ion concentration, pH and population density. Temperature regulates virulence genes which serve as an ‘on-off’ switch in a manner unique from the more general heat-shock response [38]. Pathogens living inside and outside of the host’s body react to changes in environment by synthesizing gene products that are needed for pathogen survival and growth. The transcription of specific virulence genes in the pathogens are usually induced at higher temperatures (37-41°C) [39]. Coronatine is a chlorosis-inducing phytotoxin produced by Pst. In vitro studies showed that the cma operon involved in COR synthesis in P. syringae pv. glycinea PG4180 was expressed in a temperature-dependent manner, with highest rates at 18°C and lowest activity at 28°C. Expression of cma::egfp in PG4180 was dependent on temperature in minimal medium and also inside the plant tissue [22]. Therefore, temperature affects disease resistance through genetic regulation and activation of defense components.
PTI, ETI, RNA silencing and temperature
The immune signaling pathway found in Arabidopsis is influenced by different environmental factors such as temperature.
The ability of Pst to cause disease susceptibility in Arabidopsis has made it a suitable model for plant-pathogen interaction studies [40]. Results showed that increased temperature has a negative effect on the expression of both COR-related and T3SS-related genes in vitro [39]. Plants activate ETI signaling at relatively low temperatures (10~23°C), whereas they change to PTI signaling at moderately elevated temperatures (23~32°C). The Arabidopsis (Actin-related Protein 6 (ARP6)) arp6 and (Histone H2A Protein 9 (H2A9)) hta 9 hta11 mutants, phenocopying plants grown at elevated temperatures, showed increased PTI and reduced ETI responses. Pathogens multiply vigorously at elevated temperatures accompanied with increased PAMP accumulation. However, the secretion of bacterial effectors was enhanced at low temperatures. Therefore, changes in temperature have distinctive effect to specific plant defense mechanisms and respond to biological functions of pathogens [41].
Almost similar temperature changes can cause different effects in resistance against different pathogens in Table 2 which suggests that minimal common basis for temperature-mediated modulation of immune responses. Current studies displayed several effects of temperature and are beginning to unravel the molecular mechanism underlying temperature-mediated modulation of defense responses.
Temperature regulation of plant immune responses to biotrophic and hemibiotrophic pathogens can be a general phenomenon and it can be mediated by various defense signaling components or a combination of multiple factors. Temperatures ranging from 16-32°C regulate the activation of several plant defense genes. It was found that the tobacco N (Necrosis) gene fails to activate resistance against Tobacco Mosaic Virus (TMV) at 30°C but conferred resistance at 23°C [42]. It was associated by the loss of HR above 27°C [41,43]. Elevated temperatures often inhibit ETI and increase RNA-silencing mediated resistance. Nuclear accumulation of TIR-NB-LRR proteins was decreased at elevated temperatures by Abscisic Acid (ABA). Elevated temperatures enhance expression of RNA components in RNA silencing machineries and expression of defense genes including R genes can be modulated by temperature [44]. Studies on elevated temperature suppression of ETI have been identified for the TNL-type of immune receptors Toll Interleukin-1 Receptor (TIR), NB-LRR-type, including the tobacco immune receptor N against TMV, but also resistance mediated by the Arabidopsis immune receptor RPS4 is suppressed at high temperature [45-47].
A recent study showed that Ubiquitin-conjugating Enzyme 13 (UBC13) is one of the key regulators of plants in response to low temperature and pathogens. The absence of UBC13 failed to activate the expression of several cold-related genes and resulted in hypersensitivity to low-temperature stress suggesting that UBC13 plays an important role in plant response to low-temperature stress. Moreover, UBC13 modulate programmed HR in response to low temperature and pathogen. Furthermore, ubc13 mutant showed low temperature- induced and salicylic acid-dependent lesion mimic phenotypes [29]. Furthermore, Hsp70 is the major target of HopI1 which is a virulence effector of Pst and HopI1 is dispensable for Pst pathogenicity, unless excess Hsp70 is present at high temperature [48]. BON1 is a negative modulator of a haplotype-specific R gene, SNC1. Plant growth homeostasis was found to regulate SNC1 function through BON1, thus playing an important role in the crossroad between the plant responses to temperature and pathogens. The bon1-1 loss-of-function mutation activates SNC1 which then activates the defense responses and compromise cell growth [49].
R genes which are involved in recognition of pathogen effectors are the primary cause of temperature-sensitive component in immune responses. This is further supported by snc1 intragenic suppressors which recovered temperature sensitivity in defense responses. Moreover, SNC1 reduced its nuclear accumulation at elevated temperature, which suppresses defense response [46]. The activation of HR by SNC1 requires nuclear localization of SNC1, however was suppressed when plants were placed at 28°C [46,50-52]. The transcript and protein levels of SNC1 are tightly controlled to prevent defense signaling in uninfected plants [53]. Studies revealed that SNC1 is indirectly negatively regulated by BONZAI 1 (BON1) protein in the plasma membrane [54]. The SNC1 protein levels are regulated by the immune adaptor Suppressor of RPS4-RLD 1 (SRFR1), the F-box protein CPR1 and several protein folding chaperones [53,55-58]. Study revealed that snc1-1 which is a gain-of-function mutation in SNC1, enhanced immune responses at 22°C and was compromised at 28°C [59,60]. Moreover, the second point mutation of snc1-4 activates the defense response at both 22°C and 28°C [43]. The snc1-4 protein accumulation at 22°C and 28°C suggests that the increased expression of SNC1 in the nucleus is necessary for its function. A point mutation was introduced into a tobacco R gene, N, also promotes defense responses at elevated temperatures and an N-mediated temperature-dependent defense response is matched with the N protein localization same with the activity of SNC1 protein. This suggests that R genes are considered necessary temperature-sensitive components of immune responses.
Studies showed that the mutations in bon1, snc1-1, srfr1-4 and cpr1-2 can be attributed to SNC1- dependent autoimmunity and was also dependent to both EDS1 and Phytoalexin Deficient 4 (PAD4) [61]. SIZ1 suppress plant defense at 22°C. SIZ1 also was activated by COP1 to regulate growth and temperature in plants and it affects PIF4 and Brassinazole-resistant 1 (BZR1) gene expression at high temperature. SNC1 independent autoimmunity is activated in siz1 mutant at high temperature, however, the dependent auto-immunity of SNC1 in the gain-offunction mutant snc1-1 is suppressed at elevated temperature [30,48]. The fast accumulation of SNC1 resulted to autoimmunity of snc1-1 mutant and the formation of curly leaves and stunted growth at 22°C [56].
PIF4 transcription is important for thermomorphogenesis and showed that suppression of auto- immunity in snc1-1 mutant depends on PIF4 at 28°C. Both increase in transcript and protein levels of PIF4 in the dark/light cycle are controlled by high ambient temperature [29,62]. COP1 is important in dark induced growth response and at high ambient temperature in a normal diurnal dark/light cycle and it regulates PIF4 translation and transcription for degradation in the nucleus [63,64].
Cladosporium fulvum is a famous fungal pathogen which interacts with the host tomato. It is a common gene-for-gene system and also various resistance (Cf) genes of tomato and specific matching fungal avirulence (Avr) genes have already been described. Research findings showed that the seedlings of tomato expressing both a Cf and the matching Avr gene died faster as a result of systemic necrosis at normal temperatures and were revived at 33°C. The Cf/Avr mediated activation of defense-related genes is reversibly suppressed at 33°C. The level of perception of the fungal a virulence factors affects the temperature sensitivity of Cf-mediated defense responses [65].
The stability of the TIR-NB protein is modulated by temperature. The chs1 mutant displayed a chilling-sensitive phenotype and defense-associated phenotypes such as accumulation of salicylic acid and hydrogen peroxide, increased cell death and PR gene expressions. The phenotypes characterized by the mutation in chs1 causes the activation of immune response under cold stress. CHS1 encodes a TIR-NB- type protein and the chilling sensitivity of chs1 was fully rescued by pad4 and eds1, but not by ndr1 (Non- Race-Specific Disease Resistance 1 (NDR1)). Low temperatures independently of the 26S proteasome pathway positively modulate the stability of CHS1 protein [66]. Aside from modulating the nucleo-cytoplasmic ratio of R or R-like proteins, increased temperature down regulate the CHS1/chs1 protein concentration, and this down regulation was responsible for temperature-dependent defense responses mediated by chs1. Moreover, the gain-of-function mutations in R proteins such as Recognition of Peronospora parasitica 4 (RPP4) and CHS3 activate immune responses and exhibit sensitive phenotypes at 16°C and were suppressed at 22°C [67-69]. Rp1 is a complex locus of maize and carries set of genes controlling race-specific resistance to rust fungus called Puccinia sorghi. The phenotype of Rp1-D21 is extremely dependent on temperature wherein the expression of the HR lesion phenotype is noticeable at lower temperatures. However, when plants were placed above 30°C, the HR phenotype expressed by Rp1- D21 was suppressed [70].
Temperature regulates R-mediated plant immune responses [5,44,71]. It was shown that high ambient temperatures reduce activation of anti-pathogen defense pathways and responses triggered by specific R genes [5,49]. Relative higher temperatures (23-32°C) enhanced PTI signaling pathway, yet when the ambient temperature is below 16°C, PTI responses are compromised and the R gene-mediated ETI signaling is activated [41]. Similar research showed that incompatible F1 hybrid plants undergo necrosis and defense responses mediated by R-like genes are activated at low temperatures [72,73]. At normal temperatures, it was found that RPP4 and rpp4 protein accumulation was found in cytoplasm and nucleus, however, the nuclear accumulation of the mutated rpp4 was reduced at low temperatures. It was also found that HSP90 regulates RPP4-mediated temperature- dependent cell death or HR and defense responses in Arabidopsis [74]. These studies explained the temperature modulation of Ror R-like-mediated defense responses.
Recent findings showed that high temperature promotes the translocation of effector proteins inside plant cells and causes the absence of Isochorismate 1 (ICS1)-mediated salicylic acid biosynthesis [9]. In Arabidopsis, an elevated temperature inhibits HR but has less influence on ETI-associated Pst virulence suppression, therefore separating these two ETI responses [75]. The activation of R genes encoding the TIR type of NB-LRR proteins is said to be responsible for the fundamental immune responses in plants. Thereby, high temperature-mediated inhibition is a well-known shared feature of R-mediated defense, specifically the R genes which encode TIR-NB-LRR proteins. The induction of PAD4, EDS1 and SA are decreased at higher temperatures [48,76].
Short-interfering RNAs or known as siRNAs are molecular markers of Posttranscriptional Gene Silencing (PTGS) and are potential tools which interfere with gene expression and prevent virus infection in different organisms. Studies showed that cassava geminivirus-induced RNA silencing was enhanced when temperature was increased from 25°C to 30°C and showed less symptomatic newly grown leaves, regardless of the virus. It was identified that temperature influences gene silencing for singlestranded DNA gemini viruses. There are possibilities that various mechanisms aside from gene silencing also regulate geminivirus accumulation at higher temperatures [77]. The Dark Green Islands (DGIs) appeared earlier in CMV-inoculated plants grown at elevated temperature compared with those at low temperature and the accumulation of virus small interfering RNAs in plants were significantly up-regulated under elevated temperatures at early stage of post treatment [32]. It was found that both virus and transgene triggered RNA silencing are compromised at low temperatures. Therefore, plants become less resistant to viruses, and RNA silencing-based phenotypes of transgenic plants are lost in low temperatures. Furthermore, the levels of virus- and transgene-derived small (21-26 nucleotides) interfering (si) RNAs are significantly diminished at low temperature. Contrastingly, the amount of siRNAs gradually increased and RNA silencing was enhanced with increasing temperature [78].
Biosynthetic signaling pathways and temperature
Salicylic acid is one of the major plant defense related hormone signaling and temperature essential for resistance against bio trophic and hemi-bio trophic pathogens [23]. In Arabidopsis, pathogen-accumulation of SA biosynthesis occurs through isochorismate pathway which includes ICS1 [79]. After the accumulation of SA, Nonexpresser of PR Genes (NPR1) increases in the nucleus then interacts with TGA and WRKY Transcription Factors (TFs) in order to regulate transcriptional reprogramming [80]. Pathogenesis-related 1 (PR1) is widely used as marker for SA signaling [81]. SA-mediated defense has been well studied as a crop protecting mechanism and one example is the SA synthetic analogue, Benzothiadiazole (BTH). It is commonly used in crop production since it provides resistance resulting in increased yield in various crops, including maize and wheat [82,83]. Moreover, the over- expression of NPR1 was found to increase disease resistance in rice [84]. It was shown also that basal defense against Pst and induction of SA during ETI was suppressed at elevated temperature [49,85]. SA also plays a vital role in PTI which has been found to be induced at elevated temperature [41,86].
A recent study showed that EDS1, PAD4, SA and Jasmonic Acid (JA) are regulated by temperature and are involved in temperature modulation but are not determinants of temperature sensitivity in the immune responses. Thereby, the inhibition of plant defense response at high temperature is mediated by other defense signaling factors or a combination of several other reasons [49]. A research was also conducted to investigate the effects of temperature on the interaction between N. tabacum and CMV. Results revealed that higher temperatures (>24°C) activated gene expression of JA pathway, however, lower temperatures ( ≤ 24°C) promote SA dependent responses [32]. JA is essential for this increase in plant defense because mutants in the and in the JA pathway cancelled the gene induction. Furthermore, SA, with an opposite activity to JA, has an accumulation rhythm waxing in midnight, which likely increases resistance against bio trophic pathogens in the morning. Besides JA and SA, a numerous metabolites showed diurnal rhythms in different parts of tobacco plants [87]. Elevated temperature induces movement of pathogen effector proteins inside plant cells and causes loss of ICS1-mediated SA biosynthesis. Results showed that a major temperature-sensitive node of SA signaling, impacting ~60% of BTH-activated genes, including ICS1 and PR1 [9].
Recent studies showed that Arabidopsis Calmodulin Binding Transcription Activator (CAMTA) factors compromised the expression of genes involved in SA-mediated immunity and SA biosynthesis in plants grown at 22°C. This suppression was overcome in plants infected by pathogens in plants exposed to 4°C for more than a week. CAMTA3-mediated repression of SA pathway genes in control plants grown at normal conditions involves the action of the N-terminal Repression Module (NRM) that acts independently of Calmodulin (CaM) binding to the IQ and CaM Binding (CaMB) domains [88].
Two int mutants revealed to have a role in ABA biosynthesis in high temperature-mediated inhibition of disease resistance conferred by SNC1 [88,89]. The int173 and int70 mutants are respectively defective in ABA2 (ABA deficient 2) and ABA3 genes that participate in ABA biosynthesis, and consequently have reduced accumulation of ABA. Reduced ABA level resulted to SNC1-mediated resistance at 28°C. In addition, ABA deficiency enhances resistance conferred by resistance to Pseudomonas syringae 4 (RPS4) at 28°C, suggesting that ABA has a role in defense responses at high temperatures. Moreover, elevated temperature and ABA significantly minimized nuclear localization of some TIR-NB-LRR R proteins like SNC1, RPS4, and N [59,89].
Leaf hyponasty, early flowering and rapid extension of plant axes are some of the few plant responses to high temperature. The list of phenotypes coincides with plant responses to effects of vegetation shade and involves the hormone called auxin. Research found that the basic Helix-Loop-Helix (bHLH) transcriptional regulator PIF4 mediates the high temperature-induced architectural adaptations of plants.
PIF4 function is involved in Gibberellin (GA) and light signaling pathways through interactions with DELLA proteins and phytochromes, respectively [90].
Thermogenesis refers to a process wherein minimal increase in ambient temperature can affect plant growth and development [91]. In Arabidopsis, it includes stem elongation and leaf elevation as well as responses to enhance leaf cooling. Thermo morphogenesis requires enhanced auxin biosynthesis, regulated by the bHLH transcription factor PIF4 and enhanced stability of the auxin co-receptor Transport Inhibitor Response 1 (TIR1), involving HSP90 [90-94]. High-temperature-mediated hypocotyl elongation involves localized changes in auxin metabolism mediated by the Indole-3 Acetic Acid (IAA)- amido synthetase called Gretchen Hagen 3 (GH3).17 [95]. These interactions of biosynthetic and immune signaling pathways with temperature revealed a complex interaction between plant growth, biotic and biotic responses in plants.
Circadian rhythm, light and plant immunity
Biological processes of various organisms are constantly changing daily and continuously affected by different environmental factors resulting to a unique biological rhythm. The internal biological rhythm within a period of time is commonly known as circadian rhythm [96]. Circadian rhythms occur even in the absence of external cues for a specific amount of time and period, known as the free running time [97]. The circadian rhythms are maintained by well-structured molecular machinery called the circadian clock. The circadian clock temporally coordinates biological processes with diurnal environmental changes, therefore enhancing overall organism development. It is well studied that a different organisms such as fungi, algae, cyanobacteria, plants, flies, birds and mammals, have their own specific circadian clock [98]. Plants are dependent on this internal clock machinery to adjust themselves with the surroundings.
The various roles of light and temperature in the modulation of plant immunity have been studied in many ways, however, the role of the clock in defense signaling remains elusive [96]. Results from scientific studies proved the fact that circadian clock plays an indispensable role in modulation plant immune system [99,100]. The clock mutants of Drosophila displayed enhanced susceptibility to pathogen invasion and a lower survival rate [101]. This reciprocal nature of immune system-clock interaction is also visible currently in plants [102]. Several studies were based on the two core clock components of the model system Arabidopsis, namely, Circadian Clock Associated 1 (CCA1) and Late elongated Hypocotyl (LHY). The involvements of these genes were studied in plant defense signaling [102,103]. The involvement of circadian rhythm in the evolution of organisms’ adaptation towards unexpected modification in the environment was explained in various clock-related research studies. Results showed that cca1 mutation compromised plant resistance to the downy mildew and CCA1 overexpression showed enhanced resistance against pathogen when infected at dusk than at dawn, showing a role of CCA1 in defense regulation at dawn [103]. Circadian regulation was also identified in the interaction between Arabidopsis and Pst strain wherein wild-type plants display greatest susceptibility at the midnight and greatest resistance at the morning. Elevated temperature affects down-regulation of immune response genes resulting to plant susceptibility against pathogens [104].
Chloroplast and light function affect plant defense responses and activation of immune responses to various pathogens is oftentimes dependent on light [107-109]. Far-red elongated Hypocotyl 3 (FHY3) and Far-red Impaired Response 1 (FAR1) are involved in regulating plant immunity. fhy3 far1 double null mutant displayed high levels of reactive oxygen species and Salicylic Acid (SA) and increased resistance to Pst infection. FAR1 and FHY3 regulate plant immunity by incorporating both chlorophyll biosynthesis and SA signaling pathway [109]. Moreover, genetic studies confirmed that light dependency of plant immunity is mediated by both photosynthesis and photoreceptor signaling [108]. A recent study showed that light is responsible for the flg22-induced accumulation of SA suggesting that light is indispensable for immune responses in plants [110]. It was found also that phyB red-light photoreceptor is a negative regulator of the PIF4 growth-promoting transcription factor, and was found to function as a thermo sensor in plants [111,112]. At high temperatures, heat inactivation of phyB results in derepression of PIF4- regulated genes, allowing plants to grow. Study also suggests that PIF4 mediates defense suppression at high temperature [51].
Moreover, silencing five individual photosystem II components in N. benthamiana increased susceptibility to turnip mosaic virus and affected photosynthesis. Similarly, resistance to bacterial pathogen Pst conferred by RPS2 and RPM1 is dependent on the red and far-red light receptors PHY (Phytochrome) A and PHYB [113]. Resistance to the blast fungus in rice is dependent on PHYA, PHYB, and PHYC. Likewise, blue light photoreceptor CRY1 (Crypto chrome 1) was known to play a vital role in RPS2-mediated resistance under continuous light [114]. Diurnal change of plant immunity against Pst and CCA1 expression were suppressed in AtCBFs knockdown line-amiR-1. The diurnal changes of endogenous melatonin may regulate corresponding changes of AtCBF/DREB1s expression and their underlying diurnal cycle of plant immunity and AtCCA1 [115]. A recent discovery showed the molecular basis underlying the implication of photoreceptor- mediated lings signaling on resistance to Arabidopsis-turnip crinkle virus TCV conferred by the R gene HRT (HR to TCV) [116-118]. Results revealed that chloroplasts are not only the major source of energy in the light but it is also host in biosynthetic pathways for the production of secondary metabolites and stress hormones and other signals which regulate gene expression and plant resistance to pathogens [119]. In addition, blue light photoreceptors such as Phototropin 1 (PHOT1), PHOT2 CRY1 and CRY2 are found important for HRT-mediated resistance to TCV. CRY2 and PHOT2 are important for stability of HRT under normal light conditions. RPS2 interacts with PHOT2, although a mutation in PHOT2 does not compromise RPS2- mediated resistance [116-118]. Furthermore, phytochromes and cyrptochromes are necessary for the activation of SAR and expression of SA-regulated genes [113,114,120]. The opening and closing of stomatal apertures and activation of defense genes are both modulated by circadian rhythms. Several PTI-related genes are activated in the morning and both PT1 and ETI were found to interact with evening elements which further explains the interplay between circadian clock and PTI [103,108,121]. Circadian rhythm is an important factor affecting physiological, biochemical and developmental processes in plants. It is evident that circadian clock rhythm and light signaling influences to plant innate immunity [122].
Biotic and abiotic stresses in plants are challenging factors affecting limited crop production and supply to solve global demand for food. This paper reveals the link between immunity, temperature, light and circadian clock responses in plants. Further genetic and molecular investigations related to biotic and abiotic regulation of plant immunity in different plant species will unravel the integration of various responses in plants. With this, we will be able to provide knowledge and predictions in the development of plant-pathogen interactions as well as the evolution of plant adaptation in changing environments. Moreover, deeper knowledge of how specific environmental factors affecting the interaction between plants and pathogens can further identify novel strategies for the development of highly resistant crops under increasingly unpredictable climatic conditions. This is also valuable for the advancement of transgenic crop production that can survive in various related stresses which is one of the solutions to global food security problems in the future.
Citation: Kim MG, Macoy DM, Lee JY, Cha JY, Kim WY (2021) Interactions between Plant Immunity, Temperature, Light, and Circadian Rhythm. J Plant Biochem Physiol. 9:262.
Received: 12-Apr-2021 Accepted: 26-Apr-2021 Published: 03-May-2021 , DOI: 10.35248/2329-9029.21.9.260
Copyright: © 2021 Kim MG, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.