Journal of Clinical and Experimental Ophthalmology
Open Access
ISSN: 2155-9570
ISSN: 2155-9570
Research Article - (2021)Volume 12, Issue 2
Introduction: To assess the long-term findings of repeated Dexamethasone (DEX) implants in patients with Retinal Vein Occlusion (RVO) with the Intraocular Pressure (IOP) measures set as the primary end point. The second end point is the anatomical and functional findings.
Methods: In this retrospective study, IOP, antiglaucoma treatment, Visual Acuity (VA), Central Macular Thickness (CMT), number of treatments, interval between treatments, and other safety outcomes data were collected at the Ophthalmology Department, Hôpital Cantonal de Fribourg during 3 years.
Results: Twenty-seven patients (28 eyes; 16 branch-RVO and 12 central-RVO) were eligible for analysis. Mean interval between diagnosis and treatment for treatment-naïve and prior-treated patients (anti-VEGF) was 23 days and 18 months, respectively. Across six repeated treatments, IOP (i.e. ≥ 21 mmHg) was elevated in 23/27 patients, 13 received IOP-lowering medications. After the first DEX implant, mean VA improved from baseline (0.8 LogMAR (20/125 Snellen Equivalent)) at month 1 (0.6 LogMAR (20/80 approximate Snellen Equivalent)) followed by a gradual decline back to baseline at month 4. Following the first implant, mean CMT decreased from baseline (604.3 μm) to 381 μm and 426.1 μm at months 1 and 4, respectively, and remained similar until end of follow-up. A similar pattern of CMT reduction occurred following implants 2 and 3. Patients received a median of two implants, with a treatment interval of approximately 5 months.
Conclusion: In a real-life clinical setting, intravitreal DEX improved VA and reduced CMT with no new safety concerns in patients with RVO. IOP elevation was transient and managed with short-term treatment with no cumulative effect on IOP elevation despite intervals <6 months , and no cases of glaucoma surgery recorded.
Ozurdex; Dexamethasone; IOP; Retina; Vein occlusion; Retrospective
Retinal Vein Occlusion (RVO) is the second most common retinal vascular disease after diabetic retinopathy [1,2]. Occlusion can occur either in the Branch Retinal Veins (BRVO) or in the Central Retinal Vein (CRVO), and Macular Edema (ME) accounts for the most frequent cause of visual impairment associated with the condition [3-5] .For many years, the treatment options for RVO were limited to laser photocoagulation and observation. Although treatment with laser photocoagulation seems to slow down the disease progression, improvement in vision is uncommon [6]. In recent years, the introduction of anti-vascular endothelial growth factors (VEGF) and sustained-release steroids have changed the treatment landscape for RVO, with variable improvements in Visual Acuity (VA) and Central Macular Thickness (CMT) [6].
Dexamethasone 0.7 mg intravitreal implant (DEX; OZURDEX®, Allergan Inc., Irvine, CA, USA), a water-soluble, long-acting sustained-release corticosteroid, has been shown to reduce oedema, fibrin deposition, capillary leakage and inflammatory cell migration by inhibiting multiple inflammatory cytokines [7]. DEX is licensed in the USA and Europe for the treatment of adult patients with ME following RVO [8,9].
The clinical efficacy and safety of DEX for ME associated with RVO were demonstrated in several studies, including randomized, double-masked, sham-controlled Phase III clinical studies, which showed improvements in Best-Corrected Visual Acuity (BCVA) and reduction of central retinal thickness with single or repeated injection [10-15]. Cataract development and increased Intraocular Pressure (IOP) are the most common side effects following DEX treatment after single or repeated implants [11]. While cataracts require surgery, increases in IOP, which typically peak 60 days after DEX implant, are usually transient and can be medically controlled in most cases [10,11,13,16]. Studies have shown that around 16.7%–38% of treated eyes require IOP-lowering medications [10,12,13].
While experience of retreatment with DEX is generally around 6 months, disease recurrence has been reported between 3 and 5 months [15-17]. Limited information is available on the efficacy and safety of repeated DEX implants in routine clinical practice, particularly at intervals shorter than 6 months. The current study reports the treatment outcomes and complications observed following repeated DEX treatment in patients with BRVO or CRVO in real-world clinical practice, with a particular focus on IOP changes and management.
Study design and patients
This was a retrospective, observational, non-comparative study designed to assess the efficacy, safety and treatment interval of DEX implant with a focus on IOP changes and management in patients with BRVO or CRVO in a single treatment centre in Switzerland. The study included adult (≥ 18 years) patients diagnosed with BRVO and CRVO who had received DEX treatment in the Ophthalmology Department of Fribourg, Hôpital Cantonal (HFR) in the past 3 years. It was conducted according to the tenets of the Declaration of Helsinki and all patients admitted to the study gave their informed consent. Treatment-naïve and prior-treated (anti-VEGF treatment) patients were included. DEX implants were administered first-line for pseudophakic eyes and patients who did not want monthly injections. Patients treated with DEX for any indication other than RVO during the same study period were excluded from the study.
End-points and assessments
During the study, data on BCVA (refraction and pinhole), CMT and IOP were collected at baseline, and at months 1 and 4 after each DEX implant. The number of DEX implants, interval between treatments and reasons for switching to another treatment was also collected during the same period. VA was measured in Snellen, then data were transformed to Logarithm of the Minimum Angle of Resolution (LogMAR) chart and IOP was measured using the Goldmann applanation tonometer. Spectral Domain Optical Coherence Tomography (SDOCT, SPECTRALIS (Heidleberg Engineering, Heidelberg, Germany)) was used to assess CMT at all-time points.
Safety parameters assessed included ocular side effects during the study, with a primary endpoint: IOP at baseline, Week 1, and months 1 and 4 after each DEX implant, as well as the need for anti-glaucoma medications or surgery and the duration of this treatment. In addition to ocular hypertension, the ocular side effects recorded included cataract accelerated progression, need for phacoemulsification, and incidence of endophthalmitis.
Data collection
The medical doctor and medical retina consultant in the Ophthalmology Department at Fribourg Canton Hospital were in charge of data collection and encoding. The encoded information was entered into a common database, where it was verified before analysis. The demographic and ocular medical data such as age, sex, treatment, ocular status (VA, state of the lens), IOP, ME and CMT were collected from the medical records of patients. Details of prior treatment need for anti-glaucoma drops and number of anti-glaucoma medication, phacoemulsification and endophthalmitis were also collected. Time windows were 1–2 weeks when data was collected, otherwise missing data (at 2 and 3 months) was specified.
Statistical analysis
Descriptive statistics are presented as mean ± standard deviation (SD) or median (interquartile range) for number of implants. The statistical analyses were performed using the R statistical software, version 3.1.3. Parameters such as VA, CMT and IOP were measured at baseline, Week 1, and months 1 and 4 and these parameters were analysed at first DEX implant to examine how they changed over time. Also, data for patients with BRVO and CRVO were analysed separately for comparison.
Study population and patient disposition
A total of 27 patients were included in this retrospective analysis and of these, one patient received bilateral treatment (28 eyes). Sixteen eyes were diagnosed with BRVO and 12 eyes were diagnosed with CRVO. The mean (SD) age of patients was 80 (9.0) years, 67% (n=18) of them were male and 57% (16 eyes) had received anti-VEGF treatment prior to entering into the study (prior-treated patients). The mean interval between diagnosis and treatment for treatment-naïve and prior-treated patients was 23 days and 18 months, respectively. Patient disposition and baseline characteristics are shown in Supplemental Table 1.
| Overall n=28 | BRVO n=16 | CRVO n=12 | ||
|---|---|---|---|---|
| VA, mean (SD), LogMAR | ||||
| Baseline (1st implant) | 0.8 (0.5) | 0.5 (0.4) | 1.2 (0.2) | |
| Month 1 (1st implant) | 0.6 (0.5) | 0.4 (0.4) | 1.1 (0.3) | |
| Month 4 (1st implant) | 0.8 (0.5) | 0.5 (0.5) | 1.1 (0.3) | |
| End of treatment* | 0.8 (0.5) | 0.5 (0.5) | 1.1 (0.3) | |
| VA, mean, approximate Snellen equivalent 18, 19 | ||||
| Baseline (1st implant) | 20/125 | 20/63 | 20/320 | |
| Month 1 (1st implant) | 20/80 | 20/50 | 20/250 | |
| Month 4 (1st implant) | 20/125 | 20/63 | 20/250 | |
| End of treatment* | 20/125 | 20/63 | 20/250 | |
| CMT (µm), mean (SD) | ||||
| Baseline (1st implant) | 604.3 (225.9) | 520.3 (166.8) | 709.3 (252.3) | |
| Month 1 (1st implant) | 381 (146.7) | 365 (157.6) | 397 (141.4) | |
| Month 4 (1st implant) | 426.1 (167.1) | 390.7 (74.1) | 477.2 (245.2) | |
| End of treatment* | 417.2 (160) | 408.4 (125.3) | 476.8 (216.7) | |
*End of treatment was defined as: last follow up, usually 4 months, after the last DEX injection, or prior to a switch to anti-VEGF
BRVO, branch retinal vein occlusion; CRVO, central retinal vein occlusion; CMT, central macular thickness; VA, visual acuity.
Table 1: VA and CMT following first DEX implant.
Intraocular pressure
IOP increase of 10−20 mmHg from baseline occurred in 14.2%, 25%, 10% and 33.3% of patients after first, second, third and fourth DEX implant, respectively. IOP increases of 5–9 mmHg after each implant are detailed in 3. Mean IOP increased 1 month after the first DEX implant (from 14.8 to 18.4 mmHg), and then returned almost to the baseline value by month 4 (14.9 mmHg). Overall, by the end of study (treatment), the IOP values were numerically higher (17.1 mmHg) than the baseline values, but not significantly different (p=0.09). Similarly, in the BRVO group, IOP increased 1 month after the DEX implant (from 14.9 to 18.9 mmHg) and returned towards baseline values by month 4 (14.1 mmHg) and end of study treatment (14.4 mmHg). Whereas in the CRVO group, the mean IOP values were numerically higher than the baseline value at all-time points. The pattern of transitory increases in IOP was similar for repeated use of DEX, with IOP falling back towards baseline by month 4 following second, third and fourth injections.
Most patients with IOP increase were managed with topical IOPlowering medications. A total of 13 patients received a mean of 2.2 (range: 1 to 3) medications with a treatment duration ranging from 2.6 to 9.1 months (mean of 5.2 months). No patient required glaucoma surgery, and only one patient needed systemic glaucoma medication.
Efficacy
Visual acuity: In the overall population, mean (SD) VA at baseline was 0.8 (0.5) LogMAR (20/125 Snellen equivalent [18,19]. After the first DEX implant, mean VA (SD) improved from baseline to 0.6 (0.5) LogMAR (20/80 Snellen equivalent [18,19]. 1 month after the implant and then returned to the baseline value at month 4 and end of treatment (i.e. at last follow up, usually 4 months, after the last DEX injection, or prior to switching) (Table 1). A similar trend was observed in patients with BRVO, where the mean (SD) VA improved 1 month after the first DEX implant to 0.4 (0.4) LogMAR (20/50 Snellen equivalent [18,19] and returned back to the baseline value of 0.5 (0.5) LogMAR (20/63 Snellen equivalent [18,19] at month 4 and end of treatment. For patients with CRVO, VA decreased from 1.2 (0.2) at baseline to 1.1 (0.3) LogMAR (20/250 Snellen equivalent [18,19] at 1 month and remained stable throughout the study.
Central macular thickness: At the first implant, mean CMT decreased from baseline (604.3 μm to 426.1 μm, at month 4 and remained at a similar level by the end of treatment (417.2 μm) (Table 1). Consistent with the overall findings, a similar trend in CMT reduction was observed in the BRVO and CRVO groups at all-time points (Table 1).
A similar pattern of reductions in CMT was seen with the second and third implants, however, by the fourth implant, baseline CMT was already lower than baseline values for previous treatments and there was an increase in mean CMT 4 months after the implant (Figure 1).
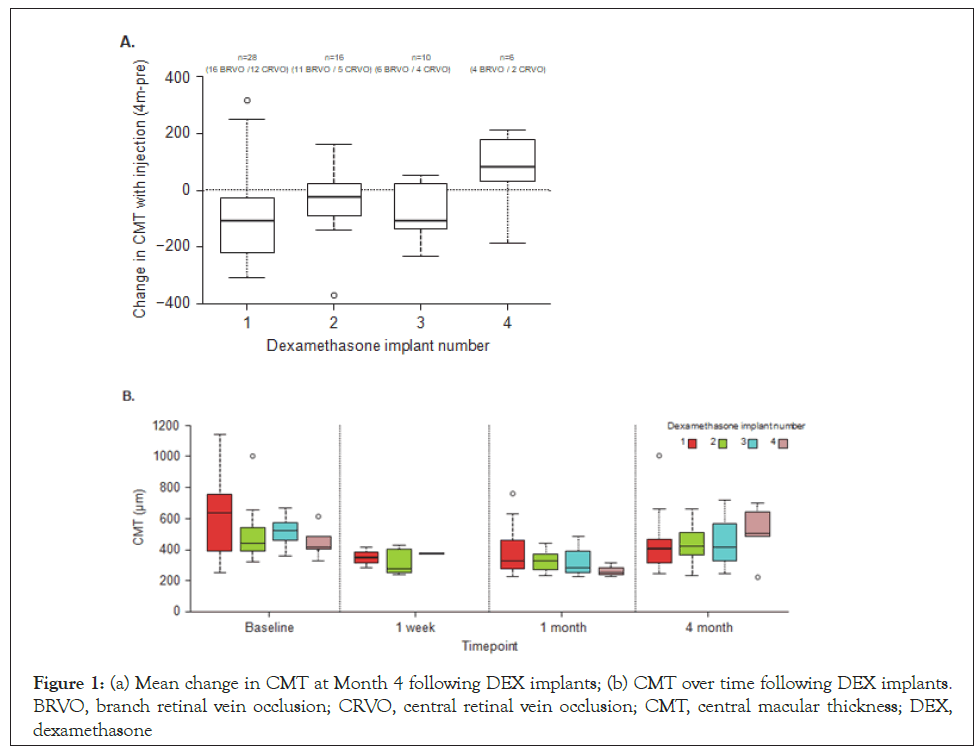
Figure 1: (a) Mean change in CMT at Month 4 following DEX implants; (b) CMT over time following DEX implants. BRVO, branch retinal vein occlusion; CRVO, central retinal vein occlusion; CMT, central macular thickness; DEX, dexamethasone
Injections: During the study, patients received a mean of 2.6 and 2.2 implants in the BRVO and CRVO groups, respectively. The median number of implants was 2. The number and timing of DEX treatments received by patients is shown in Table 2. Twelve eyes (43%) received just one DEX implant during the study and the remaining 16 eyes (57%) received repeated DEX implants (6 (21%) received two implants, 4 (14%) received three implants and 6 (21%) received ≥ 4 implants). The mean time between first and second implants was 5 months, and the interval was almost the same at subsequent implants, except between the fourth and fifth implants where the interval was 11 months (Table 2) (Figure 2).
| Injection number | Interval between injection numbers | Mean time between injection (months) | N | Discontinued DEX | Switched | Reason for switch† | Remained in study |
|---|---|---|---|---|---|---|---|
| 1 | 1-2 | 5 | 28 | 12* | 5 | 3 OHT, 2 cataract, 1 neovascularization | 16 |
| 2 | 2-3 | 5 | 16 | 6 | 2 | 2 OHT, 1 cataract | 10 |
| 3 | 3-4 | 6 | 10 | 4 | 4 | 3 OHT, 1 cataract, 2 insurance issue | 6 |
| 4 | 4-5 | 11 | 6 | 2 | 2 | 2 OHT, 1 neovascularization | 4 |
| 5 | 5-6 | 4 | 4 | 3 | NA | Endophthalmitis suspected, advanced edema with scar | 1 |
| 6 | 1 | 1 | 1 | Neovascularization | 0 |
*After the first DEX implant, 12 eyes received no further DEX treatment due to: switch to other treatments including anti-vascular endothelial
growth factor (n=5), missing data at Months 1 and 4 for newly enrolled patients (n=4), discontinued treatment (n=3; one patient due to poor
health, one patient had resolution of ME and one patient did not return to the clinic).
†A patient could have switched for more than one reason, e.g. cataract and OHT.
DEX, dexamethasone; OHT, ocular hypertension
Table 2: Injection frequency and treatment discontinuation during the study (eyes n=28).
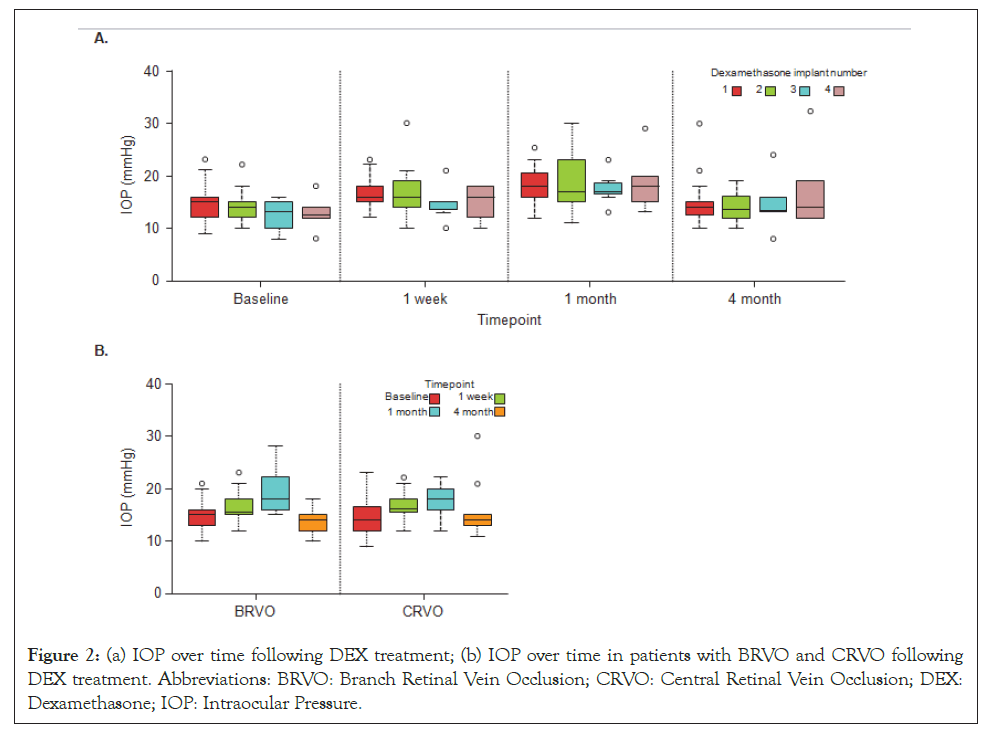
Figure 2: (a) IOP over time following DEX treatment; (b) IOP over time in patients with BRVO and CRVO following DEX treatment. Abbreviations: BRVO: Branch Retinal Vein Occlusion; CRVO: Central Retinal Vein Occlusion; DEX: Dexamethasone; IOP: Intraocular Pressure.
The reason for switch to other therapies was not always noted and is reported where available. The reasons for switch were:
• Ocular hypertension (n=10), accelerated progression of cataract (n=4),
• Neovascularization (n=3), insurance related issues (n=2), suspicion of endophthalmitis (n=1) and
• Advanced edema with scar (n=1; Table 2).
One patient refused further DEX treatment after two implants. Three patients discontinued the study having been lost to followup (after the second implant), or due to dementia (after the fifth implant) or macular scar (after the fifth implant).
Safety (except IOP): There were no cases of endophthalmitis reported during the study, although one patient with a suspected case of endophthalmitis was switched to anti-VEGF treatment after the fifth DEX implant (Table 3). Accelerated cataract formation was observed in six patients, and all needed phacoemulsification. Patients with cataract and ocular hypertension were switched to other treatments after the first, second or third implants.
| DEX1 (n=28) | DEX2 (n=16) | DEX3 (n=10) | DEX4 (n=6) | DEX5 (n=4) | DEX6 (n=1) | ||
|---|---|---|---|---|---|---|---|
| Ocular hypertension (IOP ≥ 21 mmHg) | 10 (35.7) | 8 (50.0) | 2 (20.0) | 2 (33.3) | 0 | 1 (100.0) | |
IOP increase, n (%) |
|||||||
| 5-9 mmHg | 2 (7.14) | 3 (18.7) | 2 (20.0) | 2 (33.3) | 1 (25.0) | 1 (100.0) | |
| 10-20 mmHg | 4 (14.2) | 4 (25.0) | 1 (10.0) | 2 (33.3) | 0 | 1 (100.0) | |
| IOP lowering medication, n (%) | 4 (14.2) | 4 (25.0) | 2 (20.0) | 2 (33.3) | 0 | 1 (100.0) | |
| Mean number of medication | 2.2 | 2.3 | 1.3 | 3 | NA | 1 | |
| Mean treatment duration, months | 9.1 | 5.8 | 2.6 | 3.3 | NA | 3 | |
IOP: Intraocular Pressure
Table 3: Increase in IOP following DEX treatment (measured at 7 days, 1 and 4 months) and IOP lowering medications used during the study.
In this retrospective observational study, DEX implant was administered up to six times (median of two injections) to patients with RVO. Treatment with DEX implant reduced CMT over 4 months, improved VA at 1 month following first injection, and resulted in transitory increases in IOP after each injection.
The mean VA gain of +10 ETDRS letters [18] at 1 month in this study was similar to the gain reported in the GENEVA study, where there was an approximate 10 letter improvement in BCVA from baseline at month 2 followed by a progressive decline in BCVA by the end of 6 months [10]. These findings were also similar to the gains in VA observed in other retrospective studies of DEX conducted in real-life clinical settings, where there was an improvement of 1–1.3 lines (5–6.5 approximate ETDRS letters) during 2–26 weeks [12,14,15,20]. A recent prospective observational study has also reported VA improvements of 7.8 letters with DEX implant at month 3 [15].
In the randomized controlled studies, maximum VA gains were achieved 2 months after the DEX implant, and the treatment seemed to be effective for up to 5 months [15]. In the current study, VA improved 1 month after the DEX implant, however, most patients were not checked between months 1 and 4, therefore VA was reported only at months 1 and 4. In the majority of cases, recurrence of ME followed by drop in VA was observed, which warranted retreatment.
In this study, mean CMT decreased from baseline at months 1 (−223.3 μm) and months 4 (−178.2 μm) after the first DEX implant. This reduction in CMT is comparable with the previous sham-controlled GENEVA study, where CMT reduced by 208 μm at month 3 compared with baseline [10]. Consistent with these findings, CMT reduction was similar in other observational studies of DEX administered in patients with RVO [12,14,15]. However, caution needs to be exercised when making cross-trial comparisons due to differences in data reporting at different time points and variation in study designs and patient populations.
Elevated IOP is the most frequent concern with DEX treatment. In the current study, there was a transient increase in IOP following each DEX implant; however IOP returned towards the baseline value by month 4 following the first, second, third and fourth implant. Experience across studies suggests that in most cases IOP increase is transient and can be successfully managed with topical IOP lowering medications [10,12-15]. A descriptive, retrospective evaluation of DEX treatment for indications including RVO in the SAFODEX study found no obvious increase in the risk of IOP elevation with early retreatment between the third and fourth month, compared with treatment intervals >4 months. This suggests that DEX treatment can be repeated every 4 months without added risk of increased IOP [13]. However, the SAFODEX study identified several risk factors for ocular hypertension following DEX, which included the aetiologies RVO and uveitis, younger age, male sex and pre-existing glaucoma treated with dual or triple therapy [13]. Therefore, regular IOP monitoring is required with DEX treatment, with particular attention paid to at-risk individuals [9,13].
The efficacy data from this study and other studies, both randomized and retrospective studies, suggest that an interval between treatments of less than 6 months may be needed for some patients [15-17]. In the current study, the mean time between first and second DEX implant was 5.2 months, and the interval was almost the same at subsequent treatments, except between fourth and fifth DEX implant (11 months). This interval is consistent with the 5.6-month interval reported in the large retrospective SHASTA study [14], and the 4.1 to 5-month intervals reported for patients with RVO during prospective evaluations of DEX implant [15,21].
As an observational study, there were inherent limitations. Due to the retrospective design, patients were treated according to the normal practice with no standardized assessments or visit schedules, and data were missing for some patients. For example, IOP data at 2 months post-DEX injection, when IOP has been reported to peak in previous studies, was missing in most patients in this study. Our findings are reflective of real-world practice, where patients discontinue treatment for many reasons and practitioners can see examples here of potential issues they may also face. Due to the small sample size, the study was not sufficiently powered to assess statistical significance and data were not assessed by treatment-naïve or prior-treated status, although this may be interesting for future larger studies. Due to the small sample size, data according to prior anti-VEGF-treated patients and treatment-naive patients could not be separated. The patient demographics were limited to Caucasian patients only. The IOP observed in the study eye could have been compared with the nontreated eye to correct IOP elevation for physiological fluctuations. There were no cases of pre-existing glaucoma, and there was no access to the optic nerve status or the visual field to assess risk for glaucoma conversion. The study could have collected the status of macular ischemia at baseline, as this may have an impact on VA in patients with CRVO.
Despite the study limitations, anatomical and safety outcomes were comparable with those seen in large clinical studies, providing reassurance for the applicability of DEX clinical trial findings to real-life clinical practice for patients with ME related to RVO. Published real-world clinical experience with repeated administation of DEX implants for RVO is limited, particularly in Switzerland. Our findings add to the collective experience for real-world practice, demonstrating that repeated DEX implants in shorter intervals do not lead to cumulative effects on IOP. In addition, our findings are in line with results from larger randomized studies.
This study demonstrates that repeated use of intravitreal DEX implant is effective in providing visual and anatomical benefits to patients with RVO in real-life clinical settings, with no new safety issues recorded. Increases in IOP were generally transient and manageable with topical treatment with no cases of glaucoma surgery, despite repeated DEX treatment.
The authors declare no conflict of interest other than editorial assistance in the preparation of the manuscript, which was funded by Allergan plc, Dublin, Ireland.
The authors wish to acknowledge all the patients enrolled in the study. Writing and editorial assistance was provided to the authors by Usha Gutti and Jackie Bannister from Fishawack Communications Ltd, Abingdon, UK and was funded by Allergan plc, Dublin, Ireland at the request of the investigator. All authors met the ICMJE authorship criteria. Neither honoraria nor payments were made for authorship.
No funding was received for this study.
Citation: Louati Y, Bergin C, Ezziat, Gottrau P, Vaclavik V (2021) Intraocular Pressure in Patients with Retinal Vein Occlusions Treated With Repeated Dexamethasone Intravitreal Implant: A Retrospective Analysis. J Clin Exp Ophthalmol. 12:873.
Received: 12-Feb-2021 Accepted: 26-Feb-2021 Published: 05-Mar-2021 , DOI: 10.35248/2155-9570.21.12.873
Copyright: © 2021 Louati Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.