Medicinal & Aromatic Plants
Open Access
ISSN: 2167-0412
ISSN: 2167-0412
Research Article - (2017) Volume 6, Issue 5
Boron doped hematite catalysts 10%B-90% α-Fe2O3 (10BH) and 5%B-95% α-Fe2O3 (5BH) were synthesized and characterized using XRD, BET and SEM techniques. Central composite design (CCD) matrix and response surface methodology (RSM) were applied to design experiments for evaluating the interactive effects of four operating variables [peroxide dosage (600-1200 mg/l), catalyst dosage (10-50 mg/l), pH (3-8), and reaction time (10-300 mins)] on the percentage COD reduction of tannery wastewater with initial COD of 1200 mg/l. RSM showed that the BH catalyst had better percentage COD reduction of 83% at optimum parameter values of 600 mg/l, 35 mg/l, 8 and 90 minutes for peroxide dosage, catalyst dosage, pH and time respectively. 71% COD reduction was achieved with the 5BH catalyst at optimum parameter values of 600 mg/l, 35 mg/l, 8 and 90 minutes for peroxide dosage, catalyst dosage, pH and time respectively. This study clearly showed that boron doping increased the catalytic activity of hematite as a catalyst in the Fenton oxidation system.
Keywords: Fenton oxidation; Response surface methodology; Central composite design; Chemical oxygen demand
Advanced oxidation processes (AOPs) are promising methods for treatment of recalcitrant wastewater [1]. Fenton oxidation is an AOP that utilizes the reaction of ferrous salts () with hydrogen peroxide (H2O2) to generates hydroxyl radicals (•OH) [2]. During the process, Fe2+ is oxidized to ferric ion Fe3+ in form of iron sludge which requires further removal. More so, in order to achieve acceptable reaction rates, tight working range of pH (about pH=3) is needed. Both cases pose setbacks to the homogeneous Fenton oxidation system [3]. Utilization of solid iron oxides as catalysts [4-6] instead of free iron ions as well as widening the range of working pH can prevent the set back of iron sludge formation [7]. Iron oxides are commonly found in nature and can be easily prepared in the laboratory. They are commonly considered to be nontoxic, environmentally friendly compounds similar to free iron ions, in contrast to other Fenton-like ions such as cobalt ions [7,8]. Having unique properties including high adsorptive and catalytic activities, iron oxides can be manipulated to achieve special physical and chemical structures in order to bring added advantages to the target reactions [9]. Many solid catalysts containing iron like Fe, Fe2O3, Fe3O4 and FeOOH have demonstrated to be effective in heterogeneous Fenton treatment of various organic pollutants in water [10,11]. In order to enhance their catalytic abilities for decomposing H2O2 to yield powerful radicals other elements are incorporated into iron-containing particles. Thus Luo et al. reported that the heterogeneous Fenton degradation rate of Rhodamine B using nano particle BiFeO3 catalyst was about 20 times higher than that obtained with Fe3O4 at pH of 5 at 25°C [12]. Xu and Wang [11] found that the removal rate of 4-chlorophenol in a heterogeneous Fenton reaction was 100% for CeO2/Fe3O4 composite catalyst after 120 minutes at a pH of 3, compared with only 5% and 21% for CeO2 and Fe3O4 catalysts, respectively. Other metals that were incorporated into iron based Fenton catalysts include; Mo (Tian et al.), Al (Patra et al.) and Ti (Zhong et al.) [13-15]. However, no works have investigated the incorporation of boron into iron based catalyst for heterogeneous Fenton-like reactions.
This study aimed to investigate the role of boron doping in enhancing the catalytic activity of hematite in heterogeneous Fentonlike oxidation reactions. For this purpose, 10BH and 5H catalysts were synthesized by sol-gel procedures and applied as catalysts for heterogeneous Fenton-like treatment of tannery wastewater.
Response surface methodology was used to study the influence of experimental parameters (catalyst and peroxide dosages, pH and time) on COD reduction and optimize Fenton oxidation of tannery influent using the synthesized catalysts.
Materials
The following is the list of materials used in this work. All the materials listed were obtained from Jossy chemical stores in Zaria, Nigeria and manufactured by BDH chemicals England.
1. Boric acid H3BO3 (>99.5%)
2. Citric acid monohydrate
3. Hydrogen Peroxide (30 wt.%)
4. Iron nitrate nonahydrate
5. De- ionized water.
6. Sodium hydroxide.
7. Hydrogen tetraoxosulphate (vi) acid (98.1%).
Wastewater collection
The wastewater sample was collected in plastic containers at the point of entry into the treatment plant of the Nigerian Institute of Leather and Science Technology (NILEST), Zaria, Nigeria. The wastewater was mixed with 25 ml 10% Nitric acid. It was placed in ice packed coolers to maintain low temperature of about 4°C to 10°C and transported to the environmental laboratory of the National Research Institute for Chemical Technology (NARICT), Zaria, Nigeria for analyses.
Synthesis and characterization of boron doped hematite catalysts (10BH and 5BH)
The catalysts were prepared by the sol-gel method according to the procedure similar to that followed by Zhang et al. [16]. For 10BH, 0.04 mol each of Fe(NO)3)3.9H2O and C6H8O7.H2O were dissolved in 100 ml de-ionized water in a 200 ml glass beaker and stirred on a magnetic stirrer. 0.04 mol H3BO3 was later added on continuous stirring. For 5BH the same procedure was followed with addition of 0.02 mol H3BO3. In both cases, the resulting yellowish-brown sol was heated to 80°C for 12 hours in order to induce gel formation. Annealing was then done in a muffle furnace at 600°C for 2 hours. The products were sieved using 125 μm sieve. The catalysts were abbreviated with 5BH (5% boron doped hematite) and 10BH (10% boron doped hematite). In terms of characterization, Scanning Electron Microscope was used in order to determine the morphology of the catalysts while X- Ray diffraction (XRD) analysis was used to identify their crystal structures.
The surface areas of the catalysts were determined using the Sear’s method [17]. 10 g of each catalyst was agitated in 100 ml of diluted hydrochloric acid at pH 3. 30 g of sodium chloride was added with stirring and the volume was made up to 150 ml of de-ionized water. Titration of the solution was done with 0.10 M NaOH and the volume V, needed to raise the pH from 4 to 9 was recorded. The specific surface area was calculated according to the equation.
S=32V-25 -Eq(1) Where S is in m2/g.
Experimental matrix design
The Central Composite Design (CCD) as implemented in licensed statistical software Design Expert-6.0.6 was used to design and analyze the experimental matrix. Designing the experimental matrix for the Fenton oxidation was done by varying the process parameters viz; catalyst and hydrogen peroxide dosages, reaction time and pH. Each factor was studied at two levels coded as -1 (low) and +1 (high). The response was expressed as the percentage COD reduction. The levels and ranges of the studied factors are presented in Table 1.
| Independent Variables | Coded Symbol |
Range and Level | |
|---|---|---|---|
| -1 | +1 | ||
| Catalyst dosage (mg/l) | A | 10 | 50 |
| Hydrogen peroxide dosage(mg/l) | B | 600 | 1200 |
| pH | C | 3 | 8 |
| Time (mins.) | D | 10 | 300 |
Table 1: Experimental ranges and levels of the factors used in the factorial design.
Fenton Oxidation
Fenton Oxidation reaction was carried out in a 150 ml cylindrical glass vessel. The catalyst particles were suspended in the wastewater. The reaction process was initiated with the addition of the oxidant (H2O2) under continuous stirring at 500 rpm at desired pH. Aliquots of 5 ml were taken after each run and taken for COD analyses.
Figure 1 show the XRD patterns of the 5BH and 10 BH catalysts. The peaks at Bragg angles (2θ) of 24.14°, 33.15, 40.86°,43.52° and 57.43° are due to a Fe2O3 (JCPDS card No. 73-0603). The peaks at 2θ of 18.97°, 30.10°, 38.2° and 54.3° can be attributed to cubic Fe3O4 (JCPDS Card No. 79-0417).
The Scanning electron microscopic (SEM) images of the catalysts shown in Plates I and II revealed some agglomerated particles in the case of the 5BH catalyst. However, the extent of agglomeration was observed to reduce in the case of the 10BH catalyst. Hence the latter has much smaller particle size than 5BH.
More so the measured specific surface areas of the 5BH and 10BH were found to be 163.8 m2/g and 176.6 m2/g respectively.
Batch wise studies were carried out according to the statistically designed experimental matrix shown in Table 2. Aim of which was to ascertain the whole region and obtain reaction conditions at which Fenton’s reagent consumption is minimal in the oxidation processes using the two catalysts. The dependent variable of the two processes is the percentage COD reduction represented by y10BH (using 10BH) and y5BH (using 5BH). The independent variables namely; catalyst and H2O2 dosages, pH and time were represented as x1, x2, x3, and x4 respectively. After studying the variables and their effects on the response, the parameters were estimated by the method of least squares. The response surface was then analysed in terms of the fitted surface.
| Run | A | B | C | D | Responses (%) | |||
|---|---|---|---|---|---|---|---|---|
| 10BH | 5BH | |||||||
| Exp | Pred | Exp | Pred | |||||
| 1 | 10 | 1200 | 8 | 10 | 60 | 58.79 | 60 | 55 |
| 2 | 10 | 600 | 3 | 300 | 57 | 58.75 | 55 | 55.42 |
| 3 | 30 | 900 | 5.5 | 155 | 55 | 54.42 | 55 | 55.75 |
| 4 | 50 | 1200 | 3 | 300 | 55 | 51.12 | 55 | 51.17 |
| 5 | 50 | 600 | 3 | 10 | 40 | 38.92 | 40 | 42.42 |
| 6 | 30 | 900 | 5.5 | 155 | 30 | 29.62 | 30 | 29.33 |
| 7 | 30 | 900 | 5.5 | 155 | 56 | 58.79 | 70 | 69.17 |
| 8 | 50 | 1200 | 8 | 300 | 45 | 46.25 | 45 | 51.08 |
| 9 | 10 | 1200 | 3 | 300 | 70 | 68.58 | 70 | 67.75 |
| 10 | 10 | 600 | 8 | 10 | 82 | 78.29 | 82 | 78.18 |
| 11 | 30 | 900 | 5.5 | 155 | 50 | 49.46 | 50 | 46 |
| 12 | 10 | 1200 | 8 | 300 | 55 | 55.92 | 50 | 51.42 |
| 13 | 10 | 1200 | 3 | 10 | 40 | 42.96 | 50 | 49.17 |
| 14 | 50 | 1200 | 8 | 10 | 43 | 43.42 | 43 | 46.08 |
| 15 | 10 | 600 | 8 | 300 | 50 | 48.08 | 50 | 53.42 |
| 16 | 50 | 600 | 8 | 10 | 45 | 45.29 | 45 | 45.33 |
| 17 | 10 | 600 | 3 | 10 | 50.8 | 51.79 | 48 | 49.58 |
| 18 | 50 | 1200 | 3 | 10 | 52 | 51.79 | 50 | 49.58 |
| 19 | 50 | 600 | 8 | 300 | 50 | 51.79 | 47 | 49.58 |
| 20 | 50 | 600 | 3 | 300 | 50 | 51.79 | 50 | 49.58 |
| 21 | 30 | 300 | 5.5 | 155 | 51 | 52.82 | 50 | 52.75 |
| 22 | 70 | 900 | 5.5 | 155 | 47 | 49.98 | 47 | 45.08 |
| 23 | 30 | 900 | 10.5 | 155 | 50 | 52.65 | 43 | 45.92 |
| 24 | 10 | 900 | 5.5 | 155 | 48 | 50.15 | 48 | 45.92 |
| 25 | 30 | 900 | 0.5 | 155 | 64 | 66.65 | 60 | 67.75 |
| 26 | 30 | 900 | 5.5 | 155 | 40 | 36.15 | 56 | 49.08 |
| 27 | 30 | 900 | 5.5 | 135 | 48 | 46.98 | 50 | 49.92 |
| 28 | 30 | 900 | 5.5 | 445 | 56 | 55.82 | 56 | 56.92 |
| 29 | 30 | 1500 | 5.5 | 155 | 55 | 51.4 | 45 | 48.33 |
| 30 | 30 | 900 | 5.5 | 155 | 55 | 51.4 | 55 | 48.33 |
Table 2: Experimental and predicted responses for Fenton oxidation of tannery effluent using 10BH and 5BH catalysts.
The processes were modelled using equations 2 and 3.


After estimating the main effects, the suitability of the proposed models for navigating the design space in terms of percentage COD reduction using the two catalysts were ascertained using a model adequacy test. In Tables 3 and 5 the results of the ANOVA for the proposed models for percentage COD reduction are presented. Valid model enables proper approximation of the true system. Hence the model adequacy check is necessary in validating the approximations and ensuring that violation of the least square regression assumptions is avoided [18].
| Response | Source | Sum of Squares | Degree of freedom | Mean square | F- Value | p - Value |
|---|---|---|---|---|---|---|
| % COD removal | Model | 2441.67 | 14 | 174.40 | 7.14 | 0.0004 |
| Residual | 342.08 | 14 | 24.43 | |||
| Lack of fit | 285.33 | 10 | 28.53 | 2.01 | 0.2614 | |
| Pure error | 56.75 | 4 | 14.19 | |||
| Total | 2794.17 | 29 |
Table 3: ANOVA of the quadratic model for percentage COD reduction using 5BH. R2 = 0.8771; R2adj=0.7542; Adq. prec. ratio=13.527.
| Factor | Coefficient Estimate | DF | Standard error | 95% CI Low | 95% CI High |
|---|---|---|---|---|---|
| Intercept | 48.96 | 1 | 2.04 | 44.58 | 53.34 |
| A | -1.92 | 1 | 1.01 | -4.04 | 0.25 |
| B | 0.000 | 1 | 1.01 | -2.16 | 2.16 |
| C | -4.67 | 1 | 1.01 | -6.83 | -2.50 |
| D | 1.75 | 1 | 1.01 | -0.41 | 3.91 |
| AB | -1.25 | 1 | 1.24 | -3.90 | 1.40 |
| AC | -3.37 | 1 | 1.24 | -6.03 | -0.75 |
| AD | 2.50 | 1 | 1.24 | -0.15 | 5.15 |
| BC | 6.50 | 1 | 1.24 | 3.85 | 9.15 |
| BD | -5.63 | 1 | 1.24 | -8.28 | -2.97 |
| CD | -1.50 | 1 | 1.24 | -4.15 | 1.15 |
Table 4: Estimated regression coefficients of model terms and their effects on the response for 5BH.
| Response | Source | Sum of Squares | Degree of freedom | Mean square | F-Value | p-Value |
|---|---|---|---|---|---|---|
| % COD removal | Model | 2563.71 | 10 | 256.3 | 32.9 | < 0.0001 |
| Residual | 140.25 | 18 | 7.79 | |||
| Lack of fit | 137.57 | 14 | 9.83 | 17.60 | 0.0095 | |
| Pure error | 2.68 | 4 | 0.67 | |||
| Total | 2704.97 | 29 |
Table 5: ANOVA of the 2FI model and model parameters for percentage COD reduction using 10BH. R2=0.9481; R2adj=0.9193; Adq prec ratio=27.567.
| Factor | Coefficient Estimate | DF | Standard error | 95% CI Low | 95% CI High |
|---|---|---|---|---|---|
| Intercept | 51.60 | 1 | 0.54 | 50.46 | 52.73 |
| A | -0.71 | 1 | 0.57 | -1.91 | 0.49 |
| B | -0.63 | 1 | 0.57 | -1.82 | 0.57 |
| C | -7.63 | 1 | 0.57 | -8.82 | -6.43 |
| D | 2.21 | 1 | 0.57 | 01.01 | 3.41 |
| AB | -0.81 | 1 | 0.70 | -2.28 | 0.65 |
| AC | -2.31 | 1 | 0.70 | -3.78 | -0.85 |
| AD | 2.44 | 1 | 0.70 | 0.97 | 3.90 |
| BC | 6.06 | 1 | 0.70 | 4.60 | 7.53 |
| BD | -3.69 | 1 | 0.70 | -5.15 | -2.22 |
| CD | -1.44 | 1 | 0.70 | -2.90 | 0.029 |
Table 6: Estimated regression coefficients of model terms and their effects on the response for 10BH. DF-Degrees of Freedom.
The models coefficients, effects and standard error of the factors and interactions are shown in Tables 4 and 6 for 10BH and 5BH models respectively.
From the ANOVA results (Tables 3 and 5), the models obtained were significant for the Fenton oxidative treatment of tannery wastewater. F-values of 32.9 and 7.14 were found for the percentage COD reduction using 5BH and 10BH. Models having P-values less than 0.05 indicate model significance whereas greater than 0.10 indicates that the model is not significant. In these cases (5BH and 10BH), values of
Likewise, the percentage COD reduction could well be predicted by the models. Satisfactory fits were indicated by the values of 0.8771 and 0.9481 respectively for the correlation coefficients (R2) of the 5BH and 10BH models. These signify that 87.7 and 95% respectively of the total variation in COD reduction reported were adequately represented by the two models. High values of R2 do not really indicate very good prediction as possibilities of having poor predictions of new observations or estimates of the mean response may arise. Hence a modified form of R2 referred to as R2adj is most at times employed. Values of R2 are always higher than or equal to R2adj because the R2adj adjusts the number of explanatory terms in a model. One main advantage of R2over R2adj is that addition of variables to model always results in an increase in R2value while no any significant effected is seen in the case of R2adj. Decrease may even be observed if unnecessary terms are added. For the 5BH model, the correlation coefficient values of R2=0.8771 and R2adj=0.7542 show fair agreement. In the case of the 10BH model, correlation coefficient values of R2= 0.9481 and R2adj=0.9193 show very good agreement compared to the 5BH model. This depicts that the experimental data fitted the latter model better and indicates non-inclusion of insignificant terms compared to the former. The "lack of fit F-values" of 2.01 and 17.60 for the 5BH and 10BH models respectively imply that the lack of fit is not significant relative to the pure error.
Plots of studentized residuals against predicted percentage COD reduction are shown in Figure 2 for 10BH and 5BH. The variance of original observations can be suggested to be a constant for all values of the response from the random scattering of the points instead of funnel-shaped pattern [19,20]. They also indicate the lack of need for transformation.
Figure 3 depict the plots of experimentally determined responses (actual values) against responses obtained from the developed approximating functions (predicted values) for the 10BH and 5BH models. The plots showed the existence of good agreement between the two sets of results.
The outlier plots, which determine the number of standard deviations of the actual value deviation from the predicted value for the percentage COD reduction, are shown in Figure 4. There is need to assess the outliers (i.e., The data points that lied far away from the true regression line). Data recording error or region of the independent factor variable space where the fitted model is a poor approximation to the true response surface can be determined using them [19]. In addition to that, estimates of regression coefficients can be distorted. Since the models equations were obtained using the least squares method and also the slope and intercept are sensitive to outliers. From the outlier plots, it is shown that all the standardized residual are within the range of ± 3.50 interval for the two models. This signifies that response surface approximation of the models were satisfactory and not associated with data recording error.
Effect of catalyst and hydrogen peroxide dosages
Figure 5 depicts the response surfaces showing the interaction between two variables catalyst and hydrogen peroxide dosages for the two models. In the case of H2O2 dosage, it is of significant importance to ensure that just enough amounts were introduced to sustain the reaction to completion. When in excess it contributes to COD and also have adverse effect on microorganisms when eventually discharged [21]. From the 10BH plot, it can be seen that COD reduction of 56.41% was achieved at catalyst and peroxide dosages of 10 and 600 mg/l respectively under pH and time conditions of 5.5 and 10 minutes. As the reaction time increased to 155 minutes, the COD reduction decreased to 52.49%. By increasing the pH to 8, the COD reduction achieved was 53.24%. This signifies decrease in pollutant degradation with increase in reaction time and pH. In the case of the 5BH plot, COD reduction of 55.41% was achieved at catalyst and peroxide dosages of 10 and 600 mg/L respectively under pH and time conditions of 3 and 10 minutes. As the reaction time increased to 155 minutes, the COD reduction decreased to 48.97%. By increasing the pH to 8, the COD reduction achieved was 59.40%. That signified that the 5BH catalyst had better catalytic potential at near neutral pH. In homogeneous Fenton reactions, the efficiency is being affected by solution pH mainly due to the pH induced changes in speciation of dissolved iron, [12]. But in this case (heterogeneous Fenton reaction), pH affects the mechanism of H2O2-active sites complex formation as shown in equations 4.
Fe(III)surf+H2O2→Fe(III)surf(H2O2) -Eq(4)
The complex formed was subsequently converted to Fe(II) as shown by equation 5.
Fe(III)surf (H2O2) → Fe(II)surf+HO2+H+ -Eq(5)
Equations 6-9 show the subsequent reaction mechanisms.
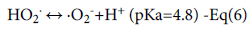

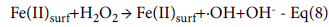
Contaminant +•OH → CO2+ H2O -Eq(9)
This is influenced by the acid/base equilibria at the catalyst surface. At lower pH the mechanism was faster hence higher rate of H2O2 decomposition on the active sites was enabled. Also the mechanism of iron (II,III) recycling due to oxidation and reduction of iron by hydroperoxyl radical and its conjugate base is favoured by lower pH. That was another factor that increased the rate of H2O2 decomposition on the catalyst’s active sites. As observed by Rusevova et al. [22], the degradation rate of phenol in a heterogeneous Fenton reaction catalysed by LaFeO3 and BiFeO3 catalysts increased significantly with decrease in pH from 7 to 5. That was also attributed to same reasons mentioned above.
Effect of Peroxide dosage and pH
The 3D interactive effects of peroxide dosage and pH for the 10BH and 5BH models are shown in Figure 6. For the former, at initial dosage of 600 mg/l and pH of 3, COD reduction of 38.72% was achieved with initial catalyst dosage of 10 mg/l and reaction time of 10 minutes. It is seen that as the catalyst dosage increased to 30 mg/l with increase in the reaction time to 155 minutes further increase in the COD reduction to 40.74% was achieved. This implies that more active sites were provided with increase in catalyst dosage for the catalytic decomposition of the peroxide to generate •OH radicals which subsequently led to increase in degradation. In the latter case, COD reduction of 41.79% was achieved at initial catalyst dosage of 10 mg/l and reaction time of 10 minutes with initial dosage of 600 mg/l and pH of 3. As the time increased to 155 minutes, 43.89% COD reduction was achieved. That implied that more active sites on the surface of the catalyst were provided for the catalytic decomposition of the peroxide to generate •OH radicals as the reaction proceeded. But increase in catalyst dosage to 50 mg/l led to decreased in COD reduction to 35.81%. The catalyst surface became a dominant sink of the •OH, hence further increase of the catalyst surface area led to reduction in concentration of the •OH radicals [22].
Effect of pH and Catalyst dosage
Figure 7 show the response surface plots of the interactions between catalyst dosage and pH for the 10BH and 5BH models respectively. For the 10BH plot, at initial catalyst dosage of 10 mg/l and pH of 3 under peroxide dosage of 600 mg/l and time 10 minutes, 36.72% COD reduction was achieved. Increase to 44.32% was observed with increase of reaction time to 155 minutes. This relates that the rate of decomposition of peroxide to generate •OH radicals increased with time. As the peroxide dosage increased to 1200 mg/l, increase in COD reduction was observed to reach 58.59%. This signifies that more •OH radicals were available for oxidation of the pollutants. In the case of the 5BH, at peroxide dosage of 600 mg/l and time 10 minutes, COD reduction of 41.79% was achieved. However, as the reaction time increased to 155 minutes, an increase of COD reduction to 43.89 was observed. This relates that the rate of decomposition of peroxide to generate •OH radicals increased with time. As the peroxide dosage was increased to 1200mg/l, increase in COD reduction to 56.54% was observed. In this case also, it signifies that more •OH radicals were generated for oxidation of the pollutants.
Effect of Time and Catalyst dosage
As depicted in Figure 8a, COD reduction of 68.32% was achieved at initial pH of 3 and peroxide dosage of 600 mg/l under constant catalyst dosage of 10 mg/l and reaction time of 10 minutes for the 10BH model However, as the peroxide dosage increased to 900mg/l, COD reduction decreased to 58.78%. This was due to the scavenging of the generated •OH radicals by the excess peroxide to form a less powerful oxidizing specie as shown in equation 10 [7].
H2O2+OH? → OOH?+ H2O -Eq(10)
The COD reduction further decreased to 42.24% as pH increased to 5.50. This can be related to the slow mechanism of H2O2 - active sites complex formation at higher pH there by leading to slow decomposition rate of the peroxide. That subsequently led to low production of •OH for contaminant degradation [22].
For the 5BH model, COD reduction of 55.95% was achieved at initial pH of 3 and peroxide dosage of 600 mg/l under constant catalyst dosage of 10 mg/l and reaction time of 10 minutes. Increase in peroxide dosage to 900 mg/l led to COD reduction decrease to 54.04%. That was also due to the scavenging of the generated •OH radicals by the excess peroxide to form a less powerful oxidizing specie as shown in equation 9 [7]. Decrease in COD to 32.24% as pH increased to 5.50 was observed also. It can be related to the slow mechanism of H2O2- active sites complex formation at higher pH at explained above. Hence leading to low production of •OH as a result of slow decomposition rate of the peroxide [22].
Optimization of Percentage COD reduction using 10BH and 5BH
Optimization of percentage COD reduction were carried out for the two models by multiple response method called desirability (D) function to optimize the key process parameters viz; catalyst and peroxide dosages, pH and time. The optimization was targeted at maximizing the COD reduction by setting the process parameters to desired levels. In this present study, pH and time were set to be within the studied range, whereas the catalyst and peroxide dosages were set to maximum and the percentage COD reduction was targeted to maximum within the range. The predicted optimum conditions are presented in Table 7 for both 10BH and 5BH.
In order to verify the optimization results, experiments were performed under predicted optimum conditions by the developed models. The 5BH model predicted 77.79% reduction of COD at pH of 8, reaction time of 90 minutes, hydrogen peroxide and catalyst dosages of 600 and 35 mg/l respectively. The experimental value obtained at these conditions was 71%. For the 10BH model, it predicted 87.596 % reduction of COD at pH of 8, reaction time of 90 minutes, hydrogen peroxide and catalyst dosages of 600 and 35 mg/l respectively. The experimental value obtained at these conditions was 83%. Table 7 shows that the predicted and experimented values for percentage COD reduction using the two catalysts are very close. This validates the suitability of the developed models to describe Fenton oxidation of tannery wastewater treatment using 5BH and 10BH catalysts.
| Catalyst | Catalyst dosage (mg/l) | Peroxide dosage (mg/l) | pH | Time (min) | Predicted COD Reduction (%) | Experimental COD Reduction (%) |
|---|---|---|---|---|---|---|
| 5BH | 35 | 600 | 8 | 90 | 77.80 | 71 |
| 10BH | 35 | 600 | 8 | 90 | 87.60 | 83 |
Table 7: Validation experiments conducted at conditions predicted by the models.
Response surface methodology was successfully used in optimizing the COD reduction of tannery wastewater of COD 1200 mg/l by Fenton oxidization using the synthesized boron doped hematite catalysts; 5%B-95% α-Fe2O3 (5BH) and 10%B- 90% α-Fe2O3 (10BH). Mathematical model equations were developed that adequately described the degradation processes. Optimum degradations were found to be 71 and 83 percents respectively for the 5BH and 10BH catalysts respectively, 10BH has better catalytic activity in the Fenton oxidation process than 5BH. Hence the role boron dopant in enhancing the catalytic performance of hematite catalyst in Fenton oxidative treatment of Tannery wastewater was established.
The authors wish to thank the Department of Chemical Engineering Ahmadu Bello University (A.B.U.) Zaria and Nigerian Research Institute for Chemical Technology (NARICT).