Medicinal & Aromatic Plants
Open Access
ISSN: 2167-0412
ISSN: 2167-0412
Research Article - (2020)Volume 9, Issue 6
Inhabitants of most rural communities of West Africa have limited health care facilities and walk long distances to seek medical attention when injured. This sometimes lead to loss of blood and death. To minimize loss of blood from injured individuals by clot formation, juices from plantain sheath/stem, cassava leaf and snail are applied on injured areas. The present work therefore investigated Musa paradisiaca sheath/stem, Manihot essculanta leaf and Achatina marginata juices for hemostatic property using human blood in-vitro. Screening indicated these juices contain tannins, flavonoids and other secondary metabolites. Morphological studies showed formation of a protein network on adding each of the three juices to fresh whole blood, plasma and serum respectively. The protein network formed was as a result of interactions between tannins found in the juices and proteins in the blood components. Microscopic analysis revealed aggregation of red blood cells as each of the juices was added to whole blood. High molecular weight fibrinogen found in whole blood, could have been caused by tannins and flavonoids found present in the juices. Insoluble proteins increased blood viscosity and inhibited movement of the red blood cells. Coagulatory potencies expressed in Prothrombin Times (PTs), revealed the snail juice of 0.80 mL volume had the least time for clot formation (PT=10.02 seconds), and indicated its hemostatic property. This PT was lesser compared to that of the standard, liquid plastin (PT= 11.0-12.5 seconds). As volumes of the three juices were increased from 0.8 to 1.0 mL, their PTs correspondingly decreased (p< 0.05). The snail juice of 0.9 mL volume had the least PT of 8.52 seconds. The plantain stem juice of 0.9 mL volume was next to the snail juice in order of ranking with a PT of 10.79 seconds, and lastly the cassava leaf juice of 1.0 mL volume with a PT of 14.42 seconds. Comparatively, the PTs for the plantain stem and snail juices were the same as the PT for the liquid plastin (PT=11.0 to 12.5 seconds, p<0.050), and all the three juices had PTs within the range considered for substances to be hemostatic (≤ 20.0 seconds). This demonstrated an increased in hemostasis as volumes of the juices were increased, and therefore the plantain stem, cassava leaf and snail juices possess hemostatic property. A blend of the plantain stem and snail juices (1:1) revealed a lesser PT of 18.20 seconds compared to the PT of ≤ 20.0 seconds considered for hemostatic substances. Thus the plantain stem, cassava leaf and snail juices, and the blend of the plantain stem and snail juices (1:1) investigated in the present research work demonstrated hemostasis, and possess hemostatic property. This scientific evidence supports the use of plantain stem, cassava leaf and snail juices as hemostatic substances.
Hemostasis; Human blood; Plantain sheath/stem; Cassava leaf; Snail juice; Prothrombin time
The ancient Greek referred to hemostasis as a process which stops outward flow of blood from a damaged blood vessel or cut by changing blood from a liquid to a gel form [1]. The outward flow of blood from a blood vessel or cut is life threatening for the wounded in battlefields, victims of vehicle and construction accidents. Hemostasis is crucial in these conditions not only for reduction of number of death individuals, but also for optimal recovery [2]. Though natural hemostasis is most desired, having other means of achieving this is also vital for survival in many emergency settings because without the ability to stimulate hemostasis, the end results of hemorrhaging will be great [3]. Vasoconstriction, temporary blockage of a cut by a platelet plug and blood coagulation or formation of a fibrin clot are the three major phases of hemostasis which seal a cut until tissues are repaired. These phases occur in a rapid sequence. The first phase causes constriction of blood vessels permitting less blood to be lost. The second phase causes platelets to stick together forming a temporary seal which covers the cut in the vessel wall. The third and last phase reinforces the platelet plug with fibrin threads that act as a molecular glue [4].
Hemostasis can be achieved by the use of chemical and physical agents. Chemical agents are often used topically in surgery settings to stop bleeding. Microfibrillar collagen is the most popular choice used among surgeons. It brings natural platelets together and begins the blood clotting process as it comes in contact with platelets. Physical agents are commonly used in situations where proper medical attention is not available. Soldiers used this method when someone is injured in a combat. The use of a dressing material on a bleeding wound exerts pressure that minimizes outward flow of blood and gives the system time for coagulation to start [5]. Gelatin sponge has been identified as a great hemostatic device which stops or reduces outward flow of blood when applied on a bleeding wound. This physical agent absorbs blood, allows coagulation to occur faster, and gives off chemical responses that decrease the time it takes for the hemostatic pathway to start [2]. Sutures are also often used to close open wounds, permitting injured areas to stay free from pathogens and other unwanted debris. Sutures and ties allow the skin to be joined together, reducing the surface area of wounds for platelets to start the process of hemostasis at a quicker pace [6].
The human body is unable to naturally achieve hemostasis, especially when experiencing shock, stress and bleeding disorders such as haemophilia, and requires careful regulation in order to work effectively. Several natural substances play a regulatory role in stopping the flow of blood from injured vessels in addition to the vessels themselves, platelets and clotting factors [7]. This has brought to limelight the use of plantain stem, cassava leaf and snail juices as hemostatic substances in some Ghanaian communities with limited or no healthcare facilities to prevent loss of blood from cut injuries.
Plantain (Musa paradisiaca) is a perennial tree-like herb in the family Musaceae [8]. It grows widely in the tropics and has nutritional and medicinal values which have made it an important fruit and vegetable crop of several countries [9]. Fruits, leaves, peels and roots from the plant are used to treat diarrhoea, dysentery, colitis, inflammation, pains, snakebite, and have shown anti-ulcerogenic, hypoglycaemic, hypolipidemic and antioxidant activities [10]. Flowers of plantain are used to treat bronchitis, epilepsy, leprosy, hemorrhages, hemorrhoids, insect and other stings and bites [11]. Plantain fruit peels and leaves have been used to improve epithelialization and alleviate pain in the treatment of chronic wounds. A study has shown fruit peels to have demonstrated potent antihypertensive activity in renal hypertensive rats [12]. Rubin and colleague have proven that plantain fruit liquid can be used to treat ulcer, dyspepsia and can promote cellular alteration of the mucosa, and increases synthesis of DNA without carcinogenic or mutagenic effects. Studies on its pulp have shown that it is rich in flavonoids and leucocyanidin, which have anti-inflammatory, anti-neoplastic and liver-protective properties [13]. The use of plantain stem juice to manage wounds and stop bleeding is very common in Benin and Ghana [14].
Cassava (Manihot esculenta) belongs to the family Euphorbiaceae. Its tubers are used for making fufu, garri, cassava and bread flours. Fufu in Yoruba and Akan, Akpu in Ibo are made from the fermented, boiled and pounded tubers of cassava, while garri is the fried version [15]. In recent times, there is increasing demand for its roots and leaves. In Nigeria, it is used for the treatment of ringworm, tumor, conjunctivitis, sores and abscesses. Leaves of this plant have also been used against disorders such as rheumatism, fever, headache, diarrhea, loss of appetite, and have demonstrated anti- hemorrhoid, anti-inflammatory and antimicrobial activities. A study showed that oral administration of an aqueous leaf extract to rats induced anti-inflammatory and analgesic effects [16]. The flavonoid compounds of the plants are thought to have antiinflammatory and analgesic effects. Studies conducted by Brender revealed that plant extract of cassava possessed antioxidant and antiradical properties [17]. Medicinally, the poisonous juice of this plant is boiled and given as an aperient [18]. Fresh rhizome made into a poultice is applied to sores. The plant is a folk remedy for abscesses, boils, conjunctivitis, diarrhea, dysentery, flu, hernia, inflammation, marasmus, prostatitis, snakebite, sores, spasm and swellings of testicles. The leaves can be used as a styptic, while the starch mixed with rum has been used for skin rashes [19]. Studies have shown cassava leaves contain alkaloids, flavonoids, tannins, anthraquinones, saponnins, reducing sugars and anthocyanosides [20]. Investigations have shown that cassava leaves contain tannins, oxalate, phytate, and trypsin which possess medicinal properties, iron and copper minerals which prevent anemia and related conditions by contributing to human blood components [21].
Snail (Achatina marginata) is a land invertebrate animal of the Phylum Mollusca, Kingdom Animalia and Class Gastropoda [22]. It is mostly found under stones, leaves or litter of decaying organic matter during the daytime. In West Africa, its favorable habitat for survival is the forest zones where rainfall is frequent and the fringe of the derived Guinea Savannah [23]. Apart from reported high nutritional value which has made it economically important in the region, it is also used in traditional medicine [24]. Ortho-calcium phosphate extracted from the shell of snail cures kidney disease, tuberculosis, anaemia, diabetes and asthma [22]. Snail meat is rich in iron and proteins with high levels of lysine, isoleucine and phenylalanine that causes reduction in pain and blood loss during labor. Its high iron content justifies its use in the treatment of anaemia. Serotonin secreted in snails’ body is effective in the maintenance of normal behaviour after mental depression [25]. An in-vitro screening of snail bluish liquid has shown that it lacked both antimicrobial and antifungal properties but established a coagulatory effect [26]. This explains its traditional use as one of the ingredients to stop bleeding in the circumcision of male genital organs [27]. One of the tests commonly employed to determine blood clot is the prothrombin time [28,29]. Thus, the present study was designed to investigate plantain stem, cassava leaf and snail juices for hemostatic property on whole blood, plasma and serum in-vitro to authenticate folklore use.
Sample collection and authentication
Plantain sheath/stem (Musa paradisiaca) and cassava leaves (Manihot essculenta) were harvested from plants in a back-yard garden at Wasa Akropong in the Western Region of Ghana. Africa giant snails (Achatina marginata) were purchased from sellers at Bogoso market, also in the Western region of Ghana. Both the plant and animal specimens were later authenticated at the Department of Applied Biology, University for Development Studies, Ghana by Dr. Isaac Sackey.
Sample preparation and extraction
Plantain sheath/ stem was thoroughly washed with tap water, sliced into smaller pieces and weighed to 200 g on an electrical balance. The weighed pieces of plantain stem were crushed with a mortar and pestle and a clean white cloth was used to squeeze out a juice from the crushed stem which was later filtered by the help of a funnel and filter paper, and then stored in a refrigerator at 4°C until required for use.
Cassava leaf was washed under running tap water to remove surface pollutants and debris, and air dried under shade at room temperature for 20 minutes. 200 g of the leaves were weighed on the balance, crushed using a mortar and pestle, and then blended in a blender. A clean white cloth was used to squeeze out a juice which was then filtered by the use of a funnel and filter paper into a clean bottle and stored at a temperature of 4°C for future use. Snails were labeled with numbers from 1 to 20 before weighing to get their respective masses which ranged from 145.0 to 287.5 g. The snail juice was obtained by breaking the bottom tip of snail’s hard shell with a knife. The juice was filtered with a filter paper and filtrate refrigerated at a temperature of 4°C for future use.
Tests for secondary metabolites
Plantain stem, cassava leaf and snail juices were screened for the presence of alkaloids, saponnins, flavonoids, tannins, phenolic, reducing sugars, terpenoids, polyphenols, glycosides, steroids, phlobatannins, anthraquinones, coumarins polyuronoids and anthracenosides using methods employed by Edoga et al. and Kubmarawa et al. [30,31].
Preparation of blood samples
Blood samples were obtained from five human volunteers of ages 16 to 42 years by venipuncture. Blood was collected in an empty tube and a tube containing 1 mL of 0.1M trisodium citrate. Contents of the tubes were properly mixed to avoid foaming. Blood was centrifuged at 4000 rpm for 15 minutes and with the help of a Pasteur pipette, the supernatant plasma was gently separated from the serum and used immediately.
Morphological evaluation
Action of plantain stem, cassava leaf and snail juices on serum, plasma and whole blood was macroscopically and microscopically observed in the laboratory of Wasa Akropong Government Hospital, Western Region, Ghana. Macroscopic analysis was carried out on serum, plasma and whole blood before and after addition of the juices. Also, a slide of 0.2 mL of each juice and 0.1 mL of each blood component was prepared for the microscopic study.
One and two-stage prothrombin time tests
A one stage prothrombin time test was carried out for each of the three different juices. 0.2 mL of fresh normal plasma obtained from the whole blood of each of the five human participants was poured into 75 × 10 mm glass test-tubes placed in a water-bath at 37°C for 1-3 minutes, and 0.4 mL of each juice was separately added to the contents of the different test tubes. A stop watch was started immediately and the time taken for the plasma to form clot was recorded. Each procedure was carried out in triplicate and the average time recorded. The two-stage Prothrombin Time (PT) test was carried out by mixing two different juices of equal volumes 0.4 mL each with 0.2 mL of the plasma and 0.2 mL of CaCl2. Experiment was conducted in triplicates and the time taken for plasma to form clot was recorded. Juice volumes were varied whiles volume of plasma was kept constant to determine the time taken for plasma to form clot.
Statistical analysis was carried out by using one way ANOVA and Descriptive Analysis (Mean ± SEM) and a Statistical Software Package for Service Solution (SPSS) version 16.0 was employed for the analysis.
Percentage (%) weights of juices obtained from snail, plantain stem and cassava leaf in decreasing order of quantities were 22.9, 17.7 and 4.02 respectively (Table 1).
Table 1: % Weights (w/w) of plantain stem,cassava leaf and snail juices.
| Juice | % w/w |
|---|---|
| Plantain stem | 17.70 |
| Cassava leaf | 4.01 |
| Snail | 22..90 |
Screening of the plantain stem, cassava leaf and snail juices showed the presence of alkaloids, saponnins, tannins, glycosides, flavonoids, reducing sugar, terpenoids, phenolic, polyphenols and anthraquinones in the juices which indicated these juices contain the fore-mentioned secondary metabolites, while phlobatannins, coumarins and steroids were absent (Table 2). The secondary metabolites found present in the juices in this work investigated have been reported in other documented works to exhibit varied biochemical and pharmacological actions [32]. Tannins have demonstrated anti-haemorrhagic property [33]. Nacoulma had stated in his research findings that flavonoids reduce bleeding [34].
Table 2: Chemical constituents of plantain stem, cassava leaf and snail juices.
| Constituents | Plantain Stem | Cassava Leaf | Snail |
|---|---|---|---|
| Alkaloids | + | + | + |
| Saponnins | + | + | + |
| Tannins | + | + | + |
| Flavonoids | + | _ | + |
| Steroids | _ | _ | _ |
| Reducing sugars | + | + | + |
| Terpenoids | + | + | + |
| Phenolic | + | + | - |
| Glycosides | + | + | + |
| Polyphenols | + | + | + |
| Phlobatannins | _ | _ | _ |
| Anthraquinones | + | + | + |
| Coumarins | _ | _ | _ |
| Polyuronoids | _ | _ | + |
| Anthracenosides | _ | + | _ |
Key: += present, - = absent
Eighty percent (80%) of the human participants screened for blood groups and haemoglobin concentrations were zero positive (0+) and non-anemic (≥ 13.0 g/dl for men and ≥ 12.0 g/dl for women) (Table 3).
Table 3: Information about participants, and data for their blood samples analysed.
| Participants | A | B | C | D | E |
|---|---|---|---|---|---|
| Age (Years) | 27 | 30 | 16 | 42 | 32 |
| Gender | Male | Male | Female | Male | Female |
| Blood group | O+ve | AB+ve | O+ve | O+ve | O+ve |
| Haemoglobin concentration (g/dl) | 13.0 | 14.4 | 11.5 | 13.4 | 13.1 |
Key: +ve= Positive
Macroscopic view after addition of each of the juices of plantain stem, cassava leaf and snail to fresh whole blood, plasma and serum respectively showed a protein network that formed blood clot (Figures 1a, 1b, 1c, Table 4). The protein network and clot formed could have resulted from interactions between the plasma or platelets in fresh whole blood or its components and tannins or flavonoids in the juices. Microscopic view of the interactions between the juices and the fresh whole blood, or blood components revealed that the protein network formed could have promoted aggregation of red blood cells (Figures 2a, 2b, 2c, and Table 5). For each juice added to the fresh whole blood or plasma there were protein networks, coagulation and red blood cell aggregates that could have originated from the interactions between tannins or flavonoids in the juice and blood plasma protein. Also for each juice added to serum, a thread-like protein network was formed due to the presence of tannins (Figures 2b and Table 5). The formation of the protein network and clot when the juices were added to plasma could also be accounted for by the presence of tannins and not flavonoids since flavonoids are deactivated and prevented from forming clot in the absence of other blood components (serum).
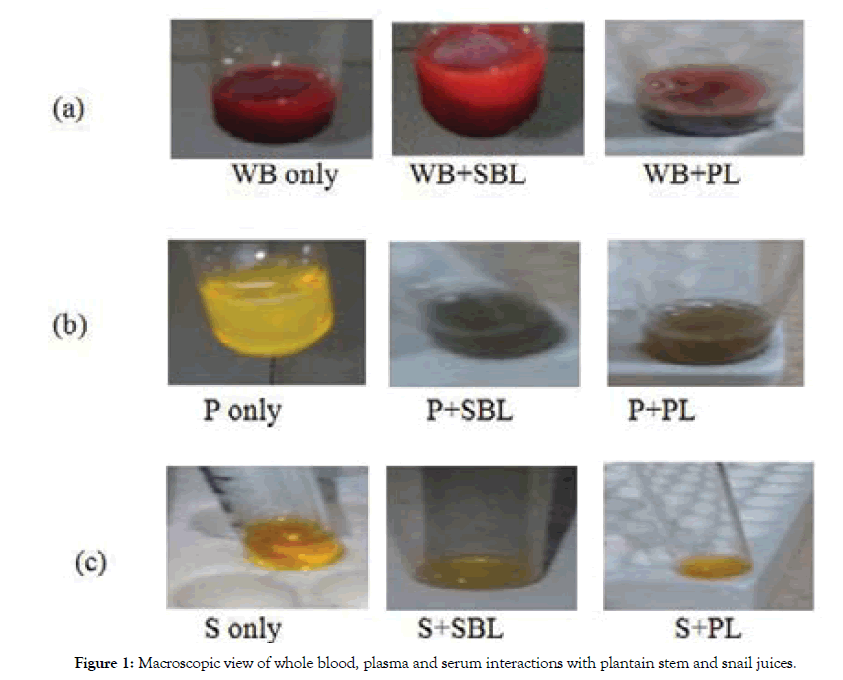
Figure 1: Macroscopic view of whole blood, plasma and serum interactions with plantain stem and snail juices.

Figure 2: Microscopic view of whole blood, plasma and serum interactions with plantain stem, cassava leaf and snail juices. Key: WB= Whole blood, P= Plasma, S= Serum, PL= Plantain Stem Juice, CL= Cassava Leaf Juice, SBL= Snail Juice
Table 4: Macroscopic view and interpretation of whole blood, plasma and serum interactions with plantain stem, cassava leaf and snail juices.
| Whole Blood/Component + Juice | Observation | Interpretation |
|---|---|---|
| WB +PL | Coagulation, and protein network formed was not pronounced | Coagulation was as a result of the presence of tannins. Soluble fibrins were converted to insoluble fibrins that formed clot. Protein network formed was not pronounced due to interferences of other blood components which made protein network not to be clearly seen. |
| WB + CL | ||
| WB + SBL | ||
| P + PL | Protein network was formed | Protein network was formed as a result of tannins’ presence and accounted for the hemostatic strength of the juices. |
| P + CL | ||
| P + SBL | ||
| S + PL | Protein network was formed | Protein network was formed due to the presence of tannins in the juices. |
| S + CL | ||
| S + SBL |
Key: WB= Whole blood, P= Plasma, S= Serum, PL= Plantain Stem Juice, CL= Cassava Leaf Juice, and SBL= Snail Juice
Table 5: Microscopic view and interpretation of whole blood, plasma and serum interactions with plantain stem, cassava leaf and snail juices.
| Whole Blood/ component | Observation | Interpretation |
|---|---|---|
| WB +PL | Aggregation of erythrocytes | Aggregation of red blood cells was due to platelets and high molecular weight fibrinogen in plasma protein which became insoluble under the action of tannins and flavonoids in the juices. |
| WB + CL | ||
| WB + SBL | ||
| P + PL | Formation of fibrin clot | Insoluble proteins, fibrin increased blood viscosity and formed clot. |
| P + CL | ||
| P + SBL | ||
| S+PLS+CL | Formation of thread-like substances | Thread-like substances formation was due to the presence of tannins in the juices |
| S+SBL | ||
Key: WB= Whole blood, P= Plasma, S= Serum, PL= Plantain stem juice, CL= Cassava leaf juice and SBL= Snail juice
The protein network and clot formed as confirmed by the microscopic view, was more pronounced or intensified for interactions between the juices and plasma than interactions between the juices and whole blood or serum. This could be explained by the fact that the separated plasma component of whole blood was present in appreciable amounts, and was precipitated by the tannins present in the juices of the plantain stem, cassava leaf and snail. Also precipitation of the plasma component of blood not separated from whole blood was inhibited by other blood components which disrupted the precipitating abilities of the tannins in the juices. Platelets and high molecular weight fibrinogen in the blood reacted with tannins and flavonoids present in the juice to form insoluble proteins (precipitates) that increased blood viscosity and inhibited movement of the red blood cells [35]. Like fibrin, it is believed that the protein network formed behaved like a net that trapped the red blood cells [36]. An increased red blood cell aggregation significantly affects blood components in the capillaries by restricting movement, and disrupts blood flow [35,37]. It causes a reduced time for clot formation. This is in line with the least time that it took the snail juice to form clot with plasma (prothrombin time=10.02 seconds) in the present studies. Next to the snail juice in order of ranking was the plantain stem juice (prothrombin time=20.71 seconds), and lastly the cassava leaf juice (prothrombin time=33.43 seconds) (Table 6). Thus the snail juice exhibited the strongest hemostatic ability on the plasma protein, and was the most active of the three juices (p< 0.05). Higher amounts of tannins in the snail juice than the plantain stem and cassava leaf juices could have caused precipitation of the plasma protein and formation of clot within the shortest possible time.
Table 6: One -stage prothrombin time (in seconds) for plantain stem, cassava leaf and snail juices mixed with plasma.
| Participants | A | B | C | D | E |
|---|---|---|---|---|---|
| P+ Plantain | 20.71 ± 0.31 | 20.71 ± 0.35 | 20.85 ± 0.32 | 20.80 ± 0.30 | 20.75 ± 0.36 |
| P+ Cassava | 33.43 ± 0.09 | 33.44 ± 0.26 | 33.41 ± 0.05 | 33.47 ± 0.18 | 33.44 ± 0.14 |
| P + Snail | 10.02 ± 0.01 | 10.03 ± 0.02 | 10.04 ± 0.02 | 10.20 ± 0.06 | 10.17 ± 0.08 |
Key: P= plasma, volume of plasma= 0.2 mL, volume of juice = 0.8 mL
One stage Prothrombin Time (PT) test showed a significantly reduced time for clot formation as volumes of the plantain stem, cassava leaf and snail juices added to plasma were increased from 0.80 mL to 0.90 mL and 1.00 mL respectively (P<0.05). The snail juice had the least time for clot formation (prothrombin time = 8.52 seconds), and was the most effective of the three juices. It was followed by the plantain stem juice (prothrombin time=10.79 seconds) in order of effectiveness, and lastly the cassava leaf juice (Tables 7-9).
Table 7: One-stage prothrombin time (in seconds) for increasing volumes of plantain stem juice mixed with plasma.
| Plantain stem juice (mL) | 0.30 | 0.40 | 0.50 | 0.60 | 0.70 | 0.80 | 0.90 |
| Plasma (mL) | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 |
| Prothrombin time (seconds) | 68.20 | 59.60 | 49.02 | 39.4 | 29.80 | 20.71 | 10.79 |
Table 8: One-stage prothrombin time (in seconds) for increasing volumes of snail juice mixed with plasma.
| Snail juice (mL) | 0.30 | 0.40 | 0.50 | 0.60 | 0.70 | 0.80 | 0.90 |
| Plasma (mL) | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 |
| Time (Seconds) | 17.52 | 16.02 | 14.52 | 13.02 | 11.52 | 10.02 | 8.52 |
Table 9: One-stage prothrombin time (in seconds) for increasing volumes of cassava leaf juice mixed with plasma
| Cassava leaf juice (mL) | 0.30 | 0.40 | 0.50 | 0.60 | 0.70 | 0.80 | 0.90 | 1.00 |
| Plasma (mL) | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 |
| Prothrombin time | 81.40 | 71.80 | 62.20 | 52.60 | 43.30 | 33.40 | 24.04 | 14.42 |
In all, comparing the PT values for varied volumes of the plantain stem, cassava leaf and snail juices with the PT values for the hemostatic agent, liquid plastin (PT=11.0 to 12.5 seconds) and other standard hemostatics (≤ 20.0 seconds), one can say the juices demonstrated hemostatic property. ANOVA test confirmed the juices have a significant value of p=0.01 which is less than p=0.05 at 95% confidence level. Post Hoc analysis revealed significant mean values between the juices were very small (p=0.01) which is less than (p=0.05) at 95% confidence level. Thus the potential effect of the plantain, cassava leaf and snail juices is to reduce time for blood to clot in-vitro, and it is the reason for their traditional use to minimize blood loss. The use of these juices significantly reduced clotting time (P<0.05), and are well desired in cases where injuries leave the blood vessels open for blood flow.
Two-stage Prothrombin Time (PT) test revealed a blend of the plantain stem and snail juices (1: 1) as the only effective hemostatic blend that formed clot when added to plasma. Its PT value of 18.20 seconds was within the PT values of ≤ 20.0 seconds considered for a hemostatic agent (Table 10). Thus in the present work, the hemostatic property of the plantain stem, cassava leaf, snail juices, and the blend of plantain stem and snail juices could be explained by the role played by their tannins and flavonoids. Tannins transform soluble proteins into insoluble substances as they form chemical bonds with proteins [38]. Their phenyl hydroxyl groups react with strong hydrogen bonds and then with atoms of the peptide binding protein, causing blood cells to quickly join the protein network and forming cell aggregates in platelet thrombus just as in the normal process of hemostasis [39,40].
Table 10: Two - stage prothrombin time (in seconds) for plantain stem, cassava leaf and snail juices mixed with plasma.
| Plasma + Juices | Time (Seconds) |
|---|---|
| P.L + CL + P | 21.11 ± 0.16 |
| PL+ SBL + P | 18.20 ± 0.07 |
| CL + SBL + P | 21.28 ± 0.57 |
Key: PL= Plantain Stem Juice, CL= Cassava Leaf Juice, SBL= Snail Juice, P= Plasma, Volume of Plasma = 0.2 ml, and volume of each juice= 0.4 ml.
The juices obtained from plantain stem, cassava leaf and snail, and the blend of plantain stem and snail juices (1:1) demonstrated hemostasis on human whole blood and plasma, and therefore possess hemostatic property. These findings support local use of the plantain stem, cassava leaf and snail juices as hemostatics to stop outward blood flow from wounds caused by injuries.
The snail juice of volumes 0.8 and 0.9 mL, with PT values of 10.02 and 8.52 seconds respectively, plantain stem juice of volume 0.9 mL, with PT value of 10.79 seconds, and the blend of plantain stem and snail juices (1:1) of volume 0.80 mL with PT value of 18.20 seconds can be used to arrest outward blood flow from wounds and damaged blood vessels after proven to be non-toxic to humans.
The authors are thankful to authorities of Wasa Akropong Government Hospital, Western Region, Ghana for permitting the use of their laboratories to conduct this research.
The authors do not have any conflict of interest
Citation: Chi Mbatchou V, James Williams T (2020) Investigation of Hemostasis Using Plantain Sheath/Stem, Cassava Leaf and Snail Juices in Human Blood in-vitro. Med Aromat Plants (Los Angeles) 9: 362. doi: 10.35248/2167-0412.20.9.362.
Received: 30-Aug-2020 Accepted: 30-Sep-2020 Published: 07-Oct-2020 , DOI: 10.35248/2167-0412.20.9.362
Copyright: © 2020 Chi Mbatchou V, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.