Pancreatic Disorders & Therapy
Open Access
ISSN: 2165-7092
ISSN: 2165-7092
Research Article - (2021)Volume 11, Issue 2
Objective: The aim of our study is to verify the prognostic role of Computed Tomography (CT) in early (2 days after symptoms onset) and delayed (>7 days) phase of moderately severe and severe acute pancreatitis (AP) based on revised Atlanta 2012 classification (RAC) in a tertiary care Italian Hospital.
Methods: We retrospectively reviewed 1412 patient’s data, identified via ICD-9 code for AP (577.0), hospitalized from January 2006 to December 2015 in our Surgical Department of Treviso Ca’Foncello Hospital. After exclusion of patients with mild disease, we then analyze 248 patients, all with documented moderately severe and severe AP based on RAC criteria. Early and delayed CT Scan Severity Index (CTSI) in acute pancreatitis were calculated and compared with Bedside Index for Severity in Acute Pancreatitis (BISAP) score and serum C-reactive Protein levels after 48 hours (CRP-48 h).
Results: Concerning all 1412 patients, 17.5% (248 patients) presented moderately severe and severe AP. Of the 248 patients included in our analysis, 133 were male (53.63%) and 115 females (46.37%) with a mean age of 63 years old. Biliary etiology was the most common finding in 47.6% of the cases, followed by alcohol with 25%, unknown origin 15.3%, post endoscopic cholangiopancreatography (ERCP) procedure in 7.3% of the cases and miscellaneous in 4.8%. At least one CT was performed in all patients. Early and delayed CTSI score showed a statistically significant correlation at Spearman Test (p-value<0.05). CRP-48 h strongly correlates with SIRS criteria.
Discussion: Both early and delayed CTSI do not correlate with organ failure and the severity of pancreatitis. Furthermore, repeating CT scan after few days did not add any statistically significant information. BISAP showed a good predictive accuracy for moderately severe and severe AP but no correlation was found with local imaging findings.
Conclusions: Our study demonstrates that in terms of decision making, CTSI does not provide essential information in early phase, both for systemic and local complications. Other scoring systems, such as BISAP, should be used in early phase for a prognostic evaluation. As most recent guidelines suggest, local complications should be treated later rather than sooner, therefore our attitude nowadays is to delay CT scan or consider MRI as late as possible even in moderately severe and severe pancreatitis.
Acute pancreatitis; Bedside index for severity in acute pancreatitis; CT scan severity index; CT scan; Severe pancreatitis; Revised Atlanta classification
Acute pancreatitis (AP) is an inflammatory condition of the pancreas that may have various clinical presentations leading different outcomes concerning peripancreatic, pancreatic tissue and multiorgan involvement. There are no recent data on the actual prevalence of AP in Italy, but according to recent literature, AP in Europe seems to have an estimated prevalence of 10- 50/100000 and a mortality rate of 6% in the adult population [1-4]. Early identification of high-risk patients can be difficult.
Acute pancreatitis in the majority of cases (80%) is mild and self- limiting, without sequelae. Pancreatic necrosis and peripancreatic fluid collections are local complications of AP and patients with pancreatic necrosis have markedly increased morbidity and mortality [5]. Therefore, it is essential to promptly define the severity to predict prognosis in order to choose the most appropriate management strategy. It is not easy to quickly assess the severity of acute pancreatitis, this can help identifying patients with an increased risk of morbidity and mortality, thereby assisting in appropriate early triage to intensive care units and selection of patients for specific interventions. The revised 2012 Atlanta criteria for classification (RAC) of the severity of acute pancreatitis are widely accepted [6]. This revised classification defines transient multi-organs failure as an organ failure which resolves completely within 48 hours, whereas lack of resolution of organ failure is defined as persistent. The presence of persistent organ failure, usually with one or more local complications, is diagnostic for severe acute pancreatitis. The absence of organ failure without any local or systemic complications indicates mild acute pancreatitis. “Moderately severe acute pancreatitis”, is characterized by transient organ failure and/or local or systemic complications in the absence of persistent organ failure, is the new grade of severity between mild and severe introduced in the revised classification in 2012. After the first week, local complications could be documented by CT scan or MRI. CT scan is more reliable in establishing pancreatic early necrosis and peripancreatic collections while MRI appears superior in the study of the biliary tree and in finding pancreatic ducts anomalies. In 1990 Balthazar first utilized the CT severity index (CTSI) for staging the AP severity [7], over the years in 2004, Mortele and his colleagues improved this score establishing the modified version of CTSI (MCTSI) [8]. The clear association between imaging findings and the evolution course of AP is still not well established. Considering that CT is not required for diagnosis of AP and moreover is not indicated in patients clinically stable and early responder to medical therapy, recent literature highlights on the lack of utility and waste of resources in performing CT scan [9]. As for the detection of pancreatic necrosis, RAC in 2012 recommend that CT scan should be done after 5 to 7 days from symptoms onset, avoiding CT in the early phase of AP due to necrosis underestimation [6]. At present there is not a clear rule and timing for CT scan to evaluate moderately severe and severe AP. The aim of our study is to verify the prognostic role of Computer Tomography (CT) in early (3 days after hospital admission) and delayed (>6 days) phase of moderately severe and severe acute pancreatitis (AP) based on revised Atlanta 2012 classification (RAC) in a tertiary care Italian Hospital. Considering the current scenario of uncertainty regarding the execution timing of CECT, especially for moderately severe and severe pancreatitis types, we focused our analysis on this subset of critical patients. BISAP score was used, calculated as shown in Table 1, to assess the degree of severity of pancreatitis at the admittance, in consideration of the simplicity and effectiveness of the score. Using BISAP score, which is very simple to calculate, allowed us, in a retrospective setting, to calculate the score in practically all cases reducing the number of missing data. We relied on the CTSI score, listed in Tables 2 and 3, to better define the local severity of pancreatitis and to better calculate the necrosis, in order to analyze the extent of necrosis and correlations with the pancreatitis severity according to the BISAP score and the Revised Atlanta Classification (2012) [10,11].
| Bed-side index values | |
|---|---|
| BUN | BUN>25 mg/dL (8.92mmol/L) (1 point) |
| Impaired mental status | Detined as disorientation, lethargy, somnolence, coma or stupor (1 point) |
| SIRS | (systemic inflammatory response syndrome) Criteria (1 point) |
| AGE | Age>60 years old (1point) |
| Pleural effusion | Imaging study reveals pleural effusion (1 point) |
| 0-2 points=Low mortality | 3-5 points=High mortality |
Table 1: Bed-side index of severity in Acute Pancreatitis (BISAP).
| Grade | Pancreas | Score | |
|---|---|---|---|
| A | Normal | 0 | |
| B | Enlargement | 1 | |
| C | Inflammatory changes in pancreas and peripancreatic fat | 2 | |
| D | Ill-defined single peripancreatic fluid collection | 3 | |
| E | Two or more poorly defined peripancreatic fluid collections | 4 | |
Table 2: CT severity index in acute pancreatitis: Grading of pancreatitis (Balthazar score).
| Pancreatic necrosis | Score |
|---|---|
| None | 0 |
| $30% | 2 |
| >30-50% | 4 |
| >50% | 6 |
CTSI is the sum of the scores obtained with the Balthazar score and those obtained with the evaluation of pancreatic necrosis:
The maximum can be obtained is 10
Table 3: Pancreatic necrosis score.
We retrospectively reviewed 1412 patient’s data, identified via ICD- 9 code for AP (577.0), hospitalized from January 2006 to December 2015 in our Surgical Department of Treviso Ca’Foncello Hospital. After a first drop out of patients, we then analyzed 248 patients, all with documented moderately severe and severe AP according to RAC criteria. Early and delayed CTSI were calculated and compared with bedside index for severity in acute pancreatitis (BISAP) score and serum C-reactive protein levels after 48 hours (CRP-48 h). Our study flowchart and drop out patients’ features are shown in Figure 1. Uncommon cases were defined as cases in which the diagnosis of Acute Pancreatitis was in doubt or secondary to a surgical procedure and they were dropped out from the analysis. Almost all patients (87%) underwent at least two abdominopelvic CT study with 256 or 64 Siemens CT scanner with 600 hundred mg of intravenous (IV) contrast material at a flow rate of 3-4 ml/s. No oral contrast was given routinely. More than one third of patients needed another imaging after the first two CT scan during hospital stay. Considering BISAP the gold standard for the systemic involvement and CTSI for local complications, we define our primary end-point as if there is correlation between CTSI, early and delayed, versus BISAP and also the accuracy of each variable analyzed in predicting the degree of severity of pancreatitis. Secondary endpoint is how often did early and delayed CT scan lead to interventional procedure, surgical, radiological and endoscopic one. All patients were treated following Italian guidelines published in 2015 [12], consisting in early fluid resuscitation preferring crystalloids, with lactated ringer solution preferred to normal saline, and colloids. Naso-gastric suction was not routinely made, unless for gastric retention. No Routine intravenous antibiotic prophylaxis was done. Although the efficacy of protease inhibitors (PI) in AP is still a matter of controversy, in our protocol department PI were administered if symptoms onset were less than 48 h. Naso-jejunal feeding was not routinely used but just for severe and ICU needed patients. Fine needle aspiration (FNA) was radiologically done only in suspected infected necrosis cases. The reintroduction of oral nutrition was when well tolerated. According to the IAP/ APA guidelines the decision to perform ERCP was taken only if there was substantial supporting evidence of bile duct stones on biochemical (presence of cholestatic liver biochemistry) and radiological (dilated common bile duct) criteria, in severe forms with jaundice we respected the early ERCP protocol (ERCP performed within 72 hours) not in emergency setting (within 24 h), while in patients with peripancreatic collections, cholecystectomy was delayed>6 weeks.
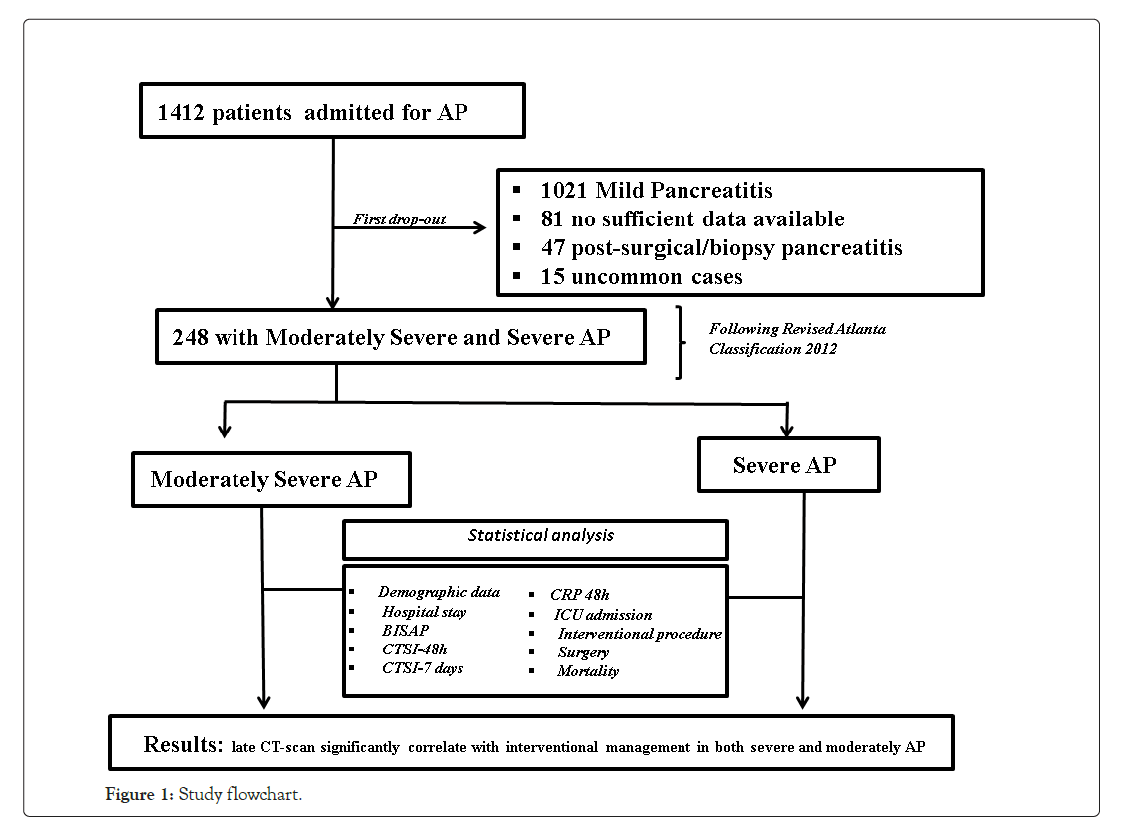
Figure 1: Study flowchart.
Statistical analysis
Bivariate relationship for categorical variables was assessed using standard parametric or non–parametric inferential tests, depending by the nature of data (χ2, t-test, Wilcoxon test for example). Spearman rank correlation analysis was used for evaluation of the correlation between each pair of scoring systems, and between each variable as shown in our results. In addition, scores’ transitions were analyzed by the use of the Markov chain R library [13]. All data were analyzed by an independent statistician. All the analyses were performed using the statistical software R [14] and, the significance was evaluated using the fixed level testing p<0.05. Kruskal Wallis (KW) test was used to better analyze correlations between Early and Late CT scan and interventional procedure to better understand which one influences more the decision to intervene or not and therefore his weight on the decision model. Missing data where very low because we decided to analyze only scores where our a priori targets for the unacceptable level of missing data were enough. Missing data vary from 2% of BISAP score to 13% of late- CTSI score. Considering a large sample, and the assumption of missing data satisfied, we decided to manage them with the Pairwise deletion eliminating information only when the particular data- point needed to test a particular assumption is missing. If there is missing data elsewhere in the data set, the existing values are used in the statistical testing.
Concerning all 1412 patients, 17.5% (248 patients) presented moderately severe and severe AP. Of the 248 patients included in our analysis, 133 were male (53.63%) and 115 females (46.37%) with a mean age of 63 years old. Biliary etiology was the most common finding in 47.6% of the cases, followed by alcohol with 25%, unknown origin 15.3%, post Endoscopic CholangioPancreatography (ERCP) procedure in 7.3% of the cases and miscellaneous in 4.8%. Patient’s data are listed in Table 4. Differences between severe and moderately severe group emerged in both early and late CTSI score, with a mean of 3.58 and 2.5 for CTSI-72 hours and 3.7 and 2.08 for CTSI-7 days. CTSI-7 days is statistical higher coherent with the severity of AP. These results are also in line with Balthazar necrosis score. As expected, the other variables in which a statistical difference was noticed are ICU admission and surgery needed. Correlations between early and late CT scan and BISAP results are shown in Tables 5 and 6. All values significantly correlated with BISAP (p-value<0.05) and the Spearman correlation coefficients are between 20-30%, hence positive, significant, but not strong correlations has been found. Moreover, there is correlation between Early and Late CT scan even in term of necrosis score at Spearman Test. If we consider CTSI score, not divided into severe and moderately severe groups, no difference between early and late CT emerged, but if we consider Balthazar and Necrosis score separately, statistical differences emerge. All other variables mentioned above and listed in tables were analyzed. No correlation between amylase and lipase values towards BISAP (p-value 0.55 and 0.50) was noticed and no relationship supports the number of recurrences and the severity of pancreatitis and/or the BISAP score (p-value>0.05). ICU admission has a strong correlation with both early and late CTSI and BISAP score (p-value<0.05). Considering all variables CRP at 48 hours strongly correlates with SIRS and infected necrosis and our cut-off value was for CRP>200 mg/L. Amylase and lipase values do not correlates with the severity of Acute Pancreatitis (p-value>0.05). At Kruskal Wallis (KW) test Late CT scan was significantly correlated to intervention procedure and these demonstrate our second end- point that in terms of weight within the decision model is the 7-day CT scan that influences any changes in therapeutic strategy. Nine patients underwent surgery, see surgical data specified in Table 7. All nine patients underwent surgery after late CT-scan imaging was done. Interventional radiological drainage was indicated in 7.25% of cases only for collections larger than 5 cm, rapidly enlarging, obstructing and suspected to be infected or when a bacteriological diagnosis was required. Necrosis without any signs of infection was just observed during the follow-up period.
| Characteristics | Moderately Severe AP | Severe AP | P value |
|---|---|---|---|
| (n=131) | (n=117) | ||
| Sex (M/F) | 70/61 | 63/54 | 1 |
| Age (years) | 62 (14-90) | 65 (49-91) | 0.2 |
| BMI (kg/m2) | 24.5 (21-36) | 24.4 (21-35) | 1 |
| Aetiology | |||
| Biliary | 76 (58.5%) | 61 (52.2%) | 0.35 |
| Alcoholic | 11 (8.5%) | 10 (8.5%) | 0.61 |
| Idiopathic | 9 (6.9%) | 13 (11.1%) | 0.34 |
| Post-CPRE | 5 (3.8%) | 3 (2.6%) | 0.57 |
| Hyperlipidemic | 2 (1.5%) | 5 (4.3%) | 0.9 |
| Autoimmune | 8 (6.2%) | 1 (0.8%) | 0.06 |
| Pancreas divisum | 3 (2.3%) | 0 | 0.09 |
| Miscellaneous | 16 (12.3%) | 24 (20.5%) | 0.1 |
| Laboratory findings | |||
| Amylase (IU/L) | 1398 (20-5250) | 1272 (19-5250) | 0.7 |
| Lipase (IU/L) | 2480 (21-5250) | 1921 (23-3330) | 0.3 |
| CRP-48h | 14 (0.1-38.43) | 21.2 (0.3-47.4) | 0.001 |
| Hospital stay (days) | 10 (5-19) | 23 (8-115) | <0.05 |
| Scoring systems: | |||
| BISAP | <0.001 | ||
| 0 | 20 (15.3%) | 5 (4.3%) | |
| I | 43 (32.8%) | 14 (12%) | |
| II | 47 (35.9%) | 31 (26.5%) | |
| III | 21 (16%) | 50 (42.7%) | |
| IV | 0 (0%) | 14 (12%) | |
| V | 0 (0%) | 3 (2.5%) | 0.0005 |
| CTSI-48h | 2.5 (0-8) | 3.58 (0-10) | |
| Balthazar | 2.2 (0-4) | 2.77 (0-4) | 0.002 |
| Necrosis | 0.25 (0-4) | 0.8 (0-6) | 0.001 |
| CTSI-7 days | 2.08 (0-10) | 3.7 (0-10) | < 0.0001 |
| Balthazar | 1.73 (0-4) | 2.43 (0-4) | 0.0007 |
| Necrosis | 0.95 (0-6) | 1.6 (0-6) | 0.0014 |
| ICU admission | 3.05% | 33.89% | < 0.0001 |
| Surgery | 0 | 7.62% | < 0.001 |
| Mortality | 1.52% | 4.27% | < 0.001 |
| IPMN | 5.34% | 1.70% | 0.78 |
| BD | 6 | 2 | |
| MT | 1 | 0 | |
| MD | 0 | 0 | |
| Pancreatic cancer | 3.05% | 1.70% | 0.003 |
Table 4: Demographic data.
| CTSI early | BISAP |
|---|---|
| Spearman rank | 0.2 |
| p-value | <0.0001 |
| Necrosis early | BISAP |
| Spearman rank | 0.21 |
| p-value | <0.0001 |
| CTSI late | BISAP |
| Spearman rank | 0.31 |
| p-value | <0.0001 |
| Necrosislate | BISAP |
| Spearman rank | 0.19 |
| p-value | <0.0001 |
Table 5: Spearman test between CTSI and BISAP.
| CTSI early | BISAP |
|---|---|
| Spearman rank | 0.747 |
| p-value | <0.0001 |
| Necrosis early | BISAP |
| Spearman rank | 0.585 |
| p-value | <0.0001 |
Table 6: Correlations between early and late CTSI.
| Patient | Types of AP | BISAP | CTSI early | CTSI late | Types of Surgery | Morbidity | Mortality |
|---|---|---|---|---|---|---|---|
| 1 | Moderate | 3 | 3 | 6 | Open cholecystectomy for gangrenous cholecystitis | + | + |
| 2 | Severe | 4 | 2 | 4 | Surgical drainage of infected collection | + | - |
| 3 | Severe | 5 | 4 | 8 | Surgical drainage of infected collection + bowel resection | + | + |
| 4 | Moderate | 2 | 2 | 6 | Surgical drainage of infected collection | + | - |
| 5 | Severe | 4 | 2 | 7 | Gastrorraphy for an associated perforated gastric ulcer | + | + |
| 6 | Severe | 4 | 2 | 6 | Surgical drainage of infected collection + bowel resection | + | + |
| 7 | Severe | 4 | 4 | 4 | Surgical drainage of infected collection | + | + |
| 8 | Severe | 3 | 2 | 5 | Open cholecystectomy for gangrenous cholecystitis | + | - |
| 9 | Severe | 5 | 8 | 8 | Surgical drainage of infected collection + laparostomy for abdominal compartment syndrome | + | + |
Table 7: Surgery data.
| Author/Year | Journal | Early CT | Late CT | Note |
|---|---|---|---|---|
| McNabb-Baltar et al. [45] | Am J Emerg Med. | - | + | Editorial |
| Chen et al. [38] | Medicine (Baltimore) | - | ? | Retrospective/EPIC score/208 patients |
| Dobbs et al. [36] | Clin Radiol. | - | + | Retrospective/100 vs 103 patients |
| Pieńkowska et al. [43] | PLoS One | + | - | Prospective/79 patients |
| Pocard et al. [34] | Diagn Interv Imaging. | + | - | Editorial |
| Dachs et al. [21] | Emerg Radiol | - | + | Retrospective/46 patients |
| Sharma et al. [37] | Gastroenterology Report | - | + | Retrospective/214 patients |
| Nistal et al. [20] | Gastroenterol Res Pract. | - | + | Retrospective/74 patients |
| Yadav et al [42] | Abdom Imaging | + | - | Prospective/57 patients |
| Watanabe et al. [41] | Pancreas | + | - | Prospective/49 patients |
Table 8: Recent literature studies.
| Author/Year | Journal/Nationality | Early CT | Late CT | Note |
|---|---|---|---|---|
| NHS guidelines/2017 | NHS guidelines/ UK guidelines | + | + | CT indicatons: Diagnostic uncertainty or failure to respond to initial treatment or clinical deterioration (Optimal timing for the CT is AT LEAST 72-96 hours after onset of symptoms) |
| Yokoe et al. [32] | J Hepatobiliary Pancreat Sci./Japanese Guidelines | + | - | CECT is recommended for identificating poorly contrasted areas of acute pancreatitis and also useful in the diagnosis of complications. However, the possibility of exacerbating pancreatitis and renal function and allergic reactions associated with the contrast must be considered. (2b) |
| Pezzilli et al. [22] | Dig Kiver Dis/ Italian Guidelines | _ | + | No less that 72 hours after the onset of AP. Evidence level 5, Recommendation grade D |
| Working group IAP/ APA [6] | Pancreatology/ International Guidelines | _ | + | Optimal timing for intial CT assessment is at least 72-96 hours after onset of AP. (GRADE 1C, strong agreement0 |
Table 9: Recent guidelines.
In current Literature there is still a debate on the opportunity of making a contrast enhanced CT scan (CECT) within 72 hours of symptoms onset. The Revised Atlanta Classification (RAC) introduces a morphological distinction between interstitialedematous and necrotizing pancreatitis. The first scenario is the most common finding and is characterized by a volumetric enlargement of the whole gland. Necrotizing form are reported to be 5-10% of all pancreatitis and usually involves a small portion of the gland evolving in late phase of the inflammatory process and this is the reason because some authors state that early CT may underestimate the extent of the necrosis [15-24]. As a matter of fact, evidence does not suggest correlation between necrosis extent, duration of symptoms and risk of infection [25]; furthermore, it is unusual to observe infected necrosis during the first week [26]. In terms of time, AP is divided into two phases by the cut-off the seventh day [27], even if both the phases are not always distinguishable. During early phase, local complications (Acute Peripancreatic Fluid Collection, APFC; Acute necrotic collection, ANC) may be present but they are not useful for determining the severity of acute pancreatitis and moreover they are not related with necrosis extension; furthermore there is no relation between local complications and organ failure [28].Erratically, late phase begins after the first week and its main features are the persistence of systemic inflammation or the development of local complications: so, late phase is related exclusively with moderate or severe AP. Even though signs of organ failure are the most useful features to define the degree of AP, after the first week it is possible to distinguish sterile from necrotizing fluid collections. So, such features must be characterized radiologically to define prognosis and treatment. The comparison of both radiological and clinical signs highlights that there is correlation with prognosis, but CTSI seems to be more suitable to predict severity of AP and local complication in the medium term, while multiparameters criteria show more accuracy in determining organ failure and systematic complications during the admission period [29-33].This latter condition shows the need to understand whether actual radiological scores are effective in predicting severity of acute pancreatitis, especially when CT scans are performed within the first 72 hours from symptoms onset. Last five years literature is listed in Table 8. In his editorial Pocard state: “In the era of the dictatorship of the ‘‘evidence-based medicine’’, it is surprising or even irritating that such a time interval for CT be not based on strong scientific data” [34]. On the other hand, daily practice guidelines have scarce adherence to best practice guidelines (Table 9). Vlada et al. showed that a large proportion of patients, 66%, underwent CT within 24 hour of admission, but only 31% of patients were imaged with contrast enhanced CT at 48–72 hours; they conclude that adherence to best practice guidelines in the treatment of severe pancreatitis is poor [35]. Our results confirm that CTSI correlates with BISAP and according to the recent literature has a good prognostic value. Moreover, even if there is a statistical correlation between CTSI, both early and late, this does not mean that they give us the same information on the local stage of pancreatic inflammation. Both early and late CTSI score have good correlation with BISAP and with the severity of pancreatitis, but late CTSI has greater accuracy for detecting pancreatic necrosis and local complications. As shown in Figure 2, analyzing differences of detected collections between early and late CT, if CTSI score in an early phase is 1 we found that in 91.7% of cases CTSI in late phase is still 1, while the highest scores at the first CT tend to decrease at the second CT. Probably at an early stage we tend to overestimate the peri-pancreatic inflammation stage, but we should consider the post-acute edema reabsorption. Concerning necrosis score (Figure 3), 37.9% of cases were not detected at early CT but if at an early stage a high necrosis score (4-6) is detected this will be confirmed almost at 100% at the second CT. At an early stage there is the risk of underestimating or missing pancreatic necrosis, but in our experience it is not true for higher necrosis score, therefore in such cases repeating a short distance CT does not add anything in terms of necrosis detection. Concerning the role of CRP, as shown in Figure 4, in our study emerged that there is strong correlation between CRP at 48 hours and SIRS, particularly for value of CRP>200 mg/L. CRP is an easily accessible marker to determine the risk for a severe outcome as well as correlates well with local complications such as necrosis but still is not helpful in predicting infected necrosis. Considering the low positive predictive value in order to identify organ failure, it cannot replace the daily appliance of the Modified Marshall scoring system for organ dysfunction, especially in patients with a CRP above 200 mg/L. Our study demonstrates that amylase and lipase values are not useful for clinical management of AP, while it is certain that patients requiring Intensive Care Unit ICU admission strongly correlate with both systemic (BISAP score) and local (CTSI score) severity regardless to CT timing. Recurrences do not correlate even if data on relapses causes should be better analyzed. At Kruskal Wallis (KW) test Late CTSI was strongly statistical associated with further interventional procedure, particularly with a surgical urgent approach (KW p-value 0.001) (Figure 5). This means that, as already emerged from other studies on this topic, CT scan execution at a late stage gives information that affects the surgical decision making more than the CTSI-72 h score and more than BISAP score. According to Dobbs et al. [36], performing CT before the sixth day of admission does not lead to earlier intervention and may offer false reassurance to clinicians. Probably new CT score and radiological findings such as EPIC (ExtraPancreatic Inflammation on CT) or perfusion pancreatic scores could offer higher sensitivity tools to better predict the course even in early acute pancreatitis phase. During these years EPIC score, assessed by the presence of ascites, pleural effusion, and retroperitoneal edema, is gaining popularity in order to predict the occurrence of organ failure in the early phase of AP [37-38]. Further studies are needed to firmly validate these new scores and to state the feasibility of daily clinical practice adoption. The power of the articles is still too poor and related to retrospective studies [39]. Moreover, adherence to best practice guidelines in the treatment of severe pancreatitis is poor Sternby et al. demonstrated that there is a significant inter-observer variation in the diagnosis of extrapancreatic necrosis and type of pancreatic collections in acute pancreatitis both in the same radiological Department and between Hub and spoke hospitals [40]. Various algorithms have been used with pancreatic perfusion CT. The single-compartment model recently has been applied to evaluate pancreatic perfusion and it allows measurements of unique tissue perfusion parameters and surrounding hemodynamic states. Watanabe et al. demonstrated that perfusion CT with single-compartment model can be useful in predicting the development of MOF in the early stage of SAP [41- 43]. In our study we have noticed that more than half of patients that already had two CT scan (early and late phase) 30-day from hospital admission had MRI and MRCP to better assess ductal obstruction, dilation, anatomical variation, or complication such as disconnected pancreatic duct syndrome. It has been reported that MR severity index (MRSI) assessed by using 0.5 Tesla (T) MR systems without contrast significantly correlate with CT severity index (CTSI), Ranson score, C-reactive protein levels, appearance of systemic complications, duration of hospitalization, and clinical outcome [44-45]. Although our study has a sample size of 248 patients, is a retrospective analysis and a non-randomized design we think that some important indications for further study emerged. A future development of our study will further investigate the role of MRI/MRCP to better find an acceptable and cost effective timing of imaging avoiding waste of time and resources. No cost analysis was performed as it would have been necessary to calculate both the direct and indirect costs, the staff involved and both procedure related costs, considering the remuneration for those in emergency setting and those inpatients setting; everything in a context such as the Italian one where the health system is completely free and the refunds mechanisms are very complex. Our goal was not to show that in most cases the execution of a CT-scanner within 72 hours from symptoms onset is not cost effective. The role of an early CT scan as recommended in most of the international guidelines should be confined only for differential diagnosis or unclear diagnosis. Excluding cases where at presentation there is a very high initial CTSI score and so late CT scan does not add information, or cases where there is diagnostic doubt, CT-scan should be delayed beyond the sixth day of hospitalization, because only at that time it would have a real impact on the daily practice decision making.
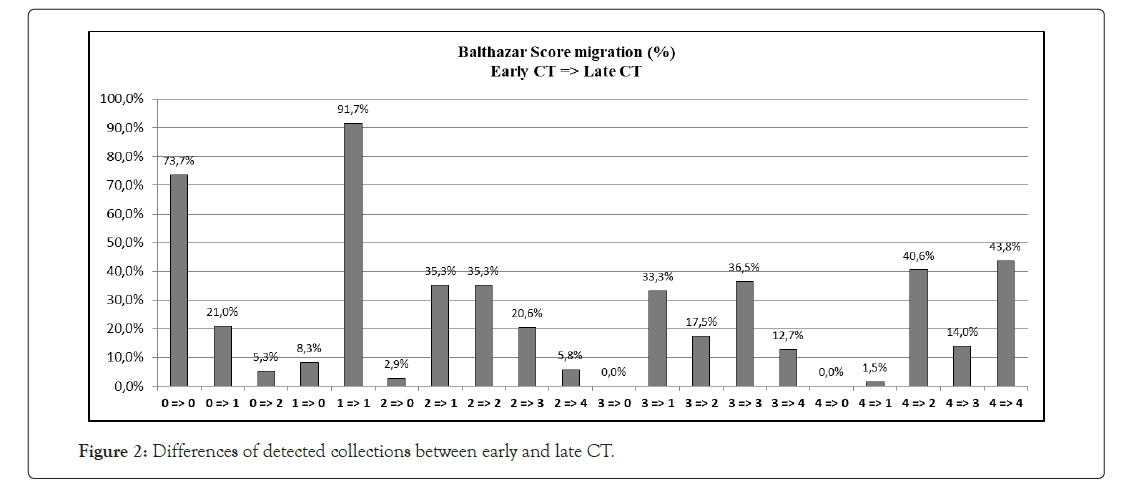
Figure 2: Differences of detected collections between early and late CT.
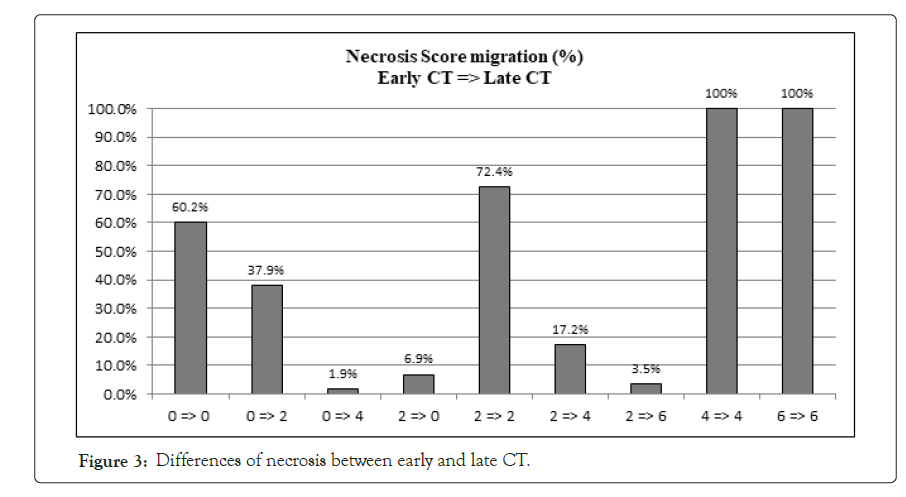
Figure 3: Differences of necrosis between early and late CT.
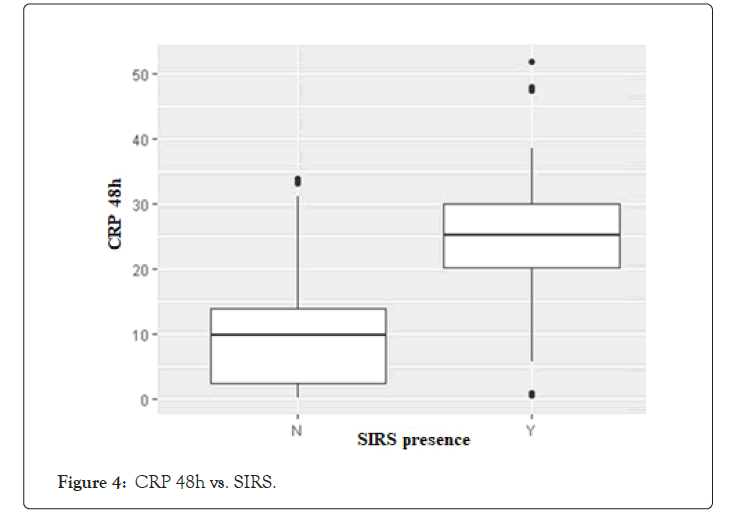
Figure 4: CRP 48h vs. SIRS.
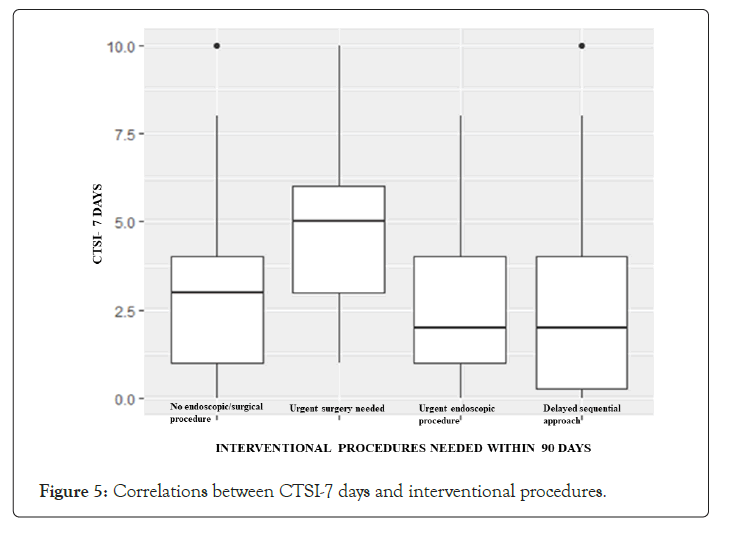
Figure 5: Correlations between CTSI-7 days and interventional procedures.
Although no definitive conclusion can be drawn from our study, we can conclude that in terms of decision making, CTSI does not provide essential information in early phase, both for systemic and local complications. Other scoring systems, such as BISAP and Modified Marshall score, should be used in early phase for a prognostic evaluation. As last guidelines suggest, local complications should be treated later rather than sooner, therefore our attitude nowadays is to delay CT scan or consider MRI as late as possible even in moderately severe and severe pancreatitis. Further studies are needed to better understand the subset of patients that would really benefit from performing a CT at an early stage. A large RCT is necessary at the moment to clarify the reasons of the failure to follow evidence-based best practice guidelines in AP.
Ethics approval was not required due to the retrospective and quality of the article design.
Authors declare that they have no competing interests.
This retrospective article is funded through internal department funds.
G.M and B.P. Contributed to the initial literature search for the content of the manuscript, filling database and design of the manuscript. T.S. and C.R. contributed significantly to the drafting of the manuscript providing materials and reviewing the English language. G.A.S. Is an independent statistician who analyzed all the data and extracted the results. G.M. and M.M gave critical review of the content and were involved in the design of the manuscript. All authors contributed to the final manuscript drafting.
Citation: Marte G, Pauletti B, Stecca T, Ruffolo C, Spedicato GA, Morana G, et al (2021) Is Early CT Scan useful in Daily Practice Decision Making for Acute Pancreatitis? Analysis of 248 Consecutive Patients in a Tertiary Care Italian Hospital. Pancreat Disord Ther. 11:211.
Received: 08-Jul-2021 Accepted: 22-Jul-2021 Published: 29-Jul-2021
Copyright: © 2021 Marte G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.