Research Article - (2015) Volume 1, Issue 1
Isolation and Evaluation of Antimicrobial Activities of Endophytic Fungal Extract from Mallotus philippinensis Muell
*Corresponding Author: Gopal Nath, Department of Microbiology, Institiute of Medical Sciences, Banaras Hindu University, UP, India, Tel: +91945-842-9841 Email:
Abstract
Mallotus philippinensis Muell an important medicinal plant in the present study i.e., endophytic extracts of different fungi associated with asymptomatic were evaluated for antimicrobial activity. This will be a new promising source of antimicrobials known as endophytes. In this preliminary study, different parts of plants viz. steam and leaves were used for isolation of endophytic fungi and fermented. The endophytic fungi were identified based on their morphology and characteristics of fungal spores. The cell free ferment broth was subjected to antimicrobial assay against different human pathogenic microbes viz. Escherichia coli, Staphylococcus aureus, E. faecalis, K .pneumoniae and P. aeruginosa and three fungal pathogenic strains of candida i.e. C. albicans, C. tropicalis and C. krusie by using standard disc diffusion method. The results showed that some of these endophytic extracts had prominent antimicrobial inhibitory effects. Among these fungal endophytes, two strains Alternaria spp., Pestalotiopsis spp., and Phompsis spp. showed the strongest antimicrobial activities against P. aeruginosa, S. aureus and E. faecalis. An endophyte Pestalotiopsis spp. had the most pronounced effect on all the Candida strains exhibited the strongest antifungal activity. This present study has proven that both these medicinal plants may be a rich source of endophytic fungi with potential to produce bioactive producing antibacterial and antifungal activities. These antipathogenic endophytes could be applied as new sources of antibiotics in agriculture and pharmaceutical industry.
Keywords: Endophytes fungus, Antibacterial, Antifungal, MIC, Alternaria, Phomopsis
Introduction
Antimicrobial resistance against many infectious disease caused by bacteria and fungus is increasing, which renders treatment of critical infectious illnesses more difficult. This leads to research of some novel antimicrobial with some different mode of action and origin too. Natural plants and their products are still to be an important source of new pharmaceutical product [1]. The probability of obtaining a novel compound is from novel source. So we selected endophytic fungus as a new source of antimicrobial agent.
German scientist Heinrich Anton De Bary [2] coined the term endophyte, used to define occurrence of bacteria or fungi insider plant tissue. Fungal endophytes are ubiquitously present to each and every plant, inhabiting the internal part of plant without causing any immediate negative effect. These microorganisms because of their potential producer of important secondary bioactive compounds representing an array of biological activities [3] and for their applications particularly in the pharmaceutical and food industries. Almost every plant species (~400, 000) harbour endophytic organisms [4].
Metabolites isolated from the fungal endophytes i.e alkaloids, terpenoids, quinines, isocoumarin derivatives, flavanoids, phenols, peptides and phenolic acids are good sources of novel antibiotics, immunosuppressant and anticancer compounds having diverse structural groups and showing antibacterial, antifungal, anticancer [5], antiviral, antioxidant, insecticide, antidiabetic and immunosuppressive activities [6]. The endophytes are also potential enzyme producers.
It is known that endophytic fungi existing in plant are the important potential sources of antimicrobial substances [7]. Some endophytic fungi possess antimicrobial activity that may be involved in a symbiotic association with a host plant. Human and plant infections caused by pathogenic microorganisms are continuing and posing a serious problem. Thus, discovery and characterization of novel and effective products for its treatment is extremely important. As these novel natural products and organisms that make them offer opportunities for innovation in drug and agrochemical discovery, this fulfills the alternative ways to control farm pests and pathogens. Endophytes offer as a promising candidate as an alternative to control animal and plant diseases [8].
Therefore, it is necessary to carry out the systematic investigation of endophytic fungi among important medicinal plants. It can be a new tool for finding new bioactive molecule against pathogenic microbes. However, few studies of antimicrobial activity of endophytic fungi have already been reported. Hence, the objective of present work is mainly focused to study the endophytic fungal diversity associated with Mallotus philippinensis Muell. Arg. collected from different ecological setups and the antibacterial and antifungal activity of selected isolated against different bacterial and fungal pathogens.
Materials and Methods
Plant collection, identification and isolation of endophytic fungus
Random healthy sampling (showing no visual disease) of root, steam and mature leaves of Mallotus philippinensis Muell. Arg. was collected from Botanical Garden, Department of Dravyaguna, Institute of Medical Sciences, Banaras Hindu University, Varanasi, India and transferred in sterile polythene bags and transported carefully to the laboratory for further isolation. The plants were identified and authenticated by Prof. R.K. Asthana Department of Botany, Banaras Hindu University, India. A reference voucher numbers RKA/BOT/ Sept.10-12 was assigned to the plant samples and preserved in Department of Botany, and also in Department of Microbiology collected from across the campus of Banaras Hindu University, Varanasi. A total 40 tissue segments, 20 each from leaf and stem representing ten different hosts from different ecological settings were sampled. Initially all the plant tissue were washed in running tap water before washing with distilled water to remove all adhere soil particles and debris. This was followed by dipping into 90% ethanol for 1 min, 5% sodium hypochlorite for 10 s, 96 % ethanol for 1 min. At last, the plant parts were rinsed three times in sterile distilled water for 2 min [9]. All sample tissues were then placed onto separate petri dishes (60 mm) containing potato dextrose agar (PDA) supplemented with 250 mg/l oxytetracycline hydrochloride (Terramycin, Pfizer) for isolating endophytic fungi. All petri dishes were sealed with sterile Parafilm™ to protect them from contamination during repeated handling while examining endophytes, and from desiccation. The plates were incubated at 26 ± 2°C and 98% relative humidity (under 12 h fluorescent light/12 h dark light), enclosed in translucent white cover plastic boxes, in a BOD cum humidity incubator for 25 days. Fungi that grew from the tissue fragments were sub cultured onto fresh PDA plates.
Partial purification of fungal extract and treatment of the fermentation broth
The isolated pure endophytic fungus was cultured in 500 ml of potato dextrose broth (PDB) of pre-fermentative flasks at 25 ± 2°C, pH 5, 120 rpm for 21 days in BOD cum incubator. The fungal biomass was separated by filtration from broth and the culture fluid was extracted twice with equal volume of ethyl acetate (EtOAc). The EtOAc fraction was dried under vacuum and the yield of crude was found up to 4.5 gm. The dried extracts obtained were subjected to antimicrobial assay against different human pathogens.
Test microorganism
A total of 4 bacterial strains viz. E. coli ATCC 25922, K. pneumonia , Enterococcus faecalis , P. aeruginosa and S. aureus (ATCC 25323) were used in the investigation for antibacterial assay and 3 different strains of Candida viz. Candida albicans , Candida tropicalis and Candida krusie for antifungal assay. All cultures were preserved at Department of Microbiology, Institute of Medical Sciences, BHU, Varanasi, India which were obtained from American Type Culture Collection (ATCC) and clinical strain. The fresh bacterial and fungal broth cultures were prepared in normal saline before the screening procedure.
Antimicrobial susceptibility test
The paper disc diffusion method was used for antimicrobial assay of fungal extract [10] according to the National Committee for Clinical Laboratory Standards [11]. MIC was determined by micro-dilution method [12] using serially diluted (2 folds) fungal extract. Crude fungal extracts were dissolved in 0.1% (v/v) DMSO. Bacterial/fungal suspension 18 hr old was adjusted to 0.5 MacFarland standards in sterile saline to achive concentration of 107 CFU/ml. These above suspension were spread on the surface of MHA/Sabouraud’s dextrose agar (SDA) (Hi- Media) agar plates for bacterial and fungal growth respectively. The inoculums was spread on the surface of the solidified media and Whatman no. 1 filter paper discs (6 mm in diameter) impregnated with the endophytic fungal extract were placed on the surface of medium and the extract was allowed to diffuse for 5 min and the plates were kept for incubation at 37°C for 24 h for bacterial culture and 72 h at 25°C for fungal culture. Standard disc of antibiotics were used as positive control. At the end of incubation, inhibitions zones were examined around the disc which if present were measured with transparent ruler in millimeters. MIC was considered as the lowest concentration producing no visible growth. This study was performed in triplicate.
Results
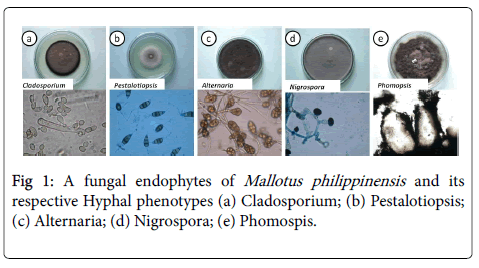
We have isolated battery of endophytic microbes from Mallotus philippinensis plants growing in several of its natural habitats, representing both fungal and actino-bacterial strains [13,14]. Several strains were isolated from surface sterilized stem tissues of the Mallotus plant, the tissue platted on a PDA plate after incubation up to two weeks, showed emergence of several endophytic hyphae (Figure 1), which were further purified on separate fresh PDA plates for pure strain (Figure 1). Endophytic fungal isolates showed microscopic features with aseptate hyphae. Sporongiophores supported by a rounded spore with flat base, in some case sporangia are completely absent. Some of the selected fungi did not produce spores when cultured on fresh media for purification, as they do not exhibit characteristic colony to distinguish. Molecular identification methods are required for further classification of some isolates.
| Microbial species | ||||||||
|---|---|---|---|---|---|---|---|---|
| Endophytic Fungus | E. coli (ATCC 35218) | S. aureus (ATCC 25323) | E. faecalis (Clinical isolate) |
S. typhi (MTCC 3216) |
P. aeruginosa (ATCC 27893) | C. albicans ATCC 90028 |
C. tropicalis ATCC 750 | C. krusie ATCC 6258 |
| Pestalotiopsismicrospora | 11.80 ± 0.26 | 12.76 ± 0.40 | - | - | - | 8.73 ± 0.75 | 11.66 ± 0.86 | 12.13 ± 0.72 |
| Nigrosporaoryzae | - | 11.80 ± 0.36 | 11.10 ± 0.32 | 10.36 ± 0.34 | 9.24 ± 0.21 | - | 11.33 ± 0.70 | - |
| Phomopsisoblonga | 10.46 ± 0.15 | - | 12.40 ± 0.88 | - | - | - | 9.40 ± 0.26 | - |
| Colletotrichumsp. | - | 10.63 ± 0.41 | - | - | - | - | 9.80 ± 0.51 | - |
| Alternariaalternata | 12.28 ± 0.24 | 10.30 ± 0.20 | - | - | 12.93 ± 0.37 | 12.26 ± 0.28 | 10.13 ± 0.55 | 10.13 ± 0.86 |
| Unidentified strain | 09.70 ± 1.34 | - | - | - | - | 11.60 ± 0.51 | - | 10.45 ± 0.53 |
| Ciprofloxacin | 33. 76 ± 0.70 | 28.53 ± 0.40 | 28.86 ± 0.46 | 29.36 ± 0.22 | 33.63 ± 0.35 | - | - | - |
| Amphotericin B | - | - | - | - | - | 15.66 ± 0.55 | 16.80 ± 0.55 | 15.93 ± 0.75 |
| DMSO | - | - | - | - | - | - | - | - |
| Diameter of inhibition zone was measured in millimeter (mm) ± SEM. | ||||||||
Table 1: Antimicrobial activity of endophytic fungal extract (zone of inhibition in mm)
| Microbial species | ||||||||
|---|---|---|---|---|---|---|---|---|
| Bacteria | Fungi | |||||||
| Endophytic Fungus | E. coli (ATCC 35218) | S. typhi (MTCC 3216) |
P. aeruginosa (ATCC 27893) | S. aureus (ATCC 25323) | E. faecalis (Clinical isolate) |
C. albicans ATCC 90028 |
C. tropicalis ATCC 750 | C. krusie ATCC 6258 |
| Pestalotiopsismicrospora | 125 | - | - | 250 | - | 125 | 125 | 62.5 |
| Nigrosporaoryzae | - | - | >500 | 125 | >500 | - | 250 | - |
| Phomopsisoblonga | 125 | - | - | - | 125 | - | 125 | - |
| Colletotrichum Sp. | - | - | - | >500 | - | - | 125 | - |
| Alternariaalternata | 62.5 | - | - | 250 | - | 125 | 250 | 250 |
| Unidentified strain | >500 | - | 125 | - | - | 250 | - | >500 |
| Ciprofloxacin | ≥ 6.25 | ≥ 6.25 | ≥ 3.12 | ≥ 6.25 | ≥ 6.25 | - | - | - |
| Amphotericin B | - | - | - | - | - | ≥ 6.25 | ≥3.12 | ≥ 3.12 |
| MIC values are reported in μg/ml | ||||||||
Table 2: Minimum inhibitory concentration (MIC) of endophytic extracts against pathogenic microbes.
All the fungal isolates from the above plants were displayed considerable antibacterial and antifungal activity against important human pathogens with inhibition zones ranging from 8.7 to 12.7 mm diameter. Among the active isolates, Alternaria sp. shows good inhibition zone i.e., 12.76 ± 0.40 mm against E.coli while 12.13 ± 0.72 mm against C. Krusie . Two isolates i.e., Alternaria and Nigrospora showed good antifungal activity against all the strains of Candida species. Some isolates showed only antibacterial or antifungal activity. After screening for antimicrobial assay, active extracts were subjected to calculated MIC determination, ranging from 7.8 to >500 μg/ml against tested microorganism.
Discussion
For the development of some novel bioactive metabolite, microbes are the best choice with special reference to microbial endophytes [15]. They are among the promising source leading to the production of novel natural bioactive compounds widely used in medicine, agriculture and industry [16]. Taxol, an anticancer drug is an excellent example supposed to occur in plant tissue [8]. Most of the isolated fungi belonged to anamorphic fungi belonging to Ascomycetes.
Mallotus philippinensis Muell having a broad spectrum of antimicrobial and other medicinal properties [12]. Fruit of Mallotus is traditionally used as antifilarial, purgative antihelmintic etc. [17] as each and every part was used in different types of medicines in Ayurveda. In this preliminary investigation, different parts of plants were used isolation of endophytic microbes. As population density of endophytic microbes seems to be highest in aerial tissue rather than underground parts. Present investigation shows that even broader flora of endophytes might be found across diverse plant species. Isolated fungus were extracted and were subjected for antimicrobial screening against human pathogens by using standard protocol indicates that the plant Mallotus philippinensis is enriched with wide variety of fungal populations which may produce some bioactive secondary metabolites responsible for positive antimicrobial activity.
Most of the isolated endophytic fungi have already been reported from some herb. However some more morphological and taxological studies will be needed to further characterize and identify these fungal species. In this preliminary study, endophytic fungi isolated from leaves and stem are used and cell free fermentation broth was collected, which was subjected to antimicrobial screening assay with use of standard protocol. Antimicrobial result revealed that S. aureus was most susceptible followed by E. coli in bacteria and C. tropicalis was most sensitive among tested fungi. Antimicrobial assay was demonstrated by formation of clear zone of inhibition around disk. This indicated that fungal islolates from Mallotus produce some active bioactive metabolites which will be further studied specifically. It also proofs that isolated endophytes may be beneficial to their host. This approach towards antibiotic resistance offers an attractive alternative [18] or supplement to disease management without any sign of negative impact or adverse effects of chemical control. Plants always serve as a source of medicinal agents along with natural products as source of all drugs [19]. Research on endophyte plant symbiosis are excellent source of natural bioactive products which will satisfy the demands of present Medical and pharmaceutical industry.
Conclusion
Present study defines and explored a scientific knowledge about the Mallotus philippinensis plant harboring natural microbes yielding useful bioactive compounds which could be great alternative to synthetic antibiotics. To the best of our knowledge, this is the first study on microbial endophytes of Mallotus philippinensis plant which will be further investigated on purification of bioactive compounds. Searching some new natural metabolites may solve the problem of antibiotic resistance with low toxicity and safe profile.
Conflict of Interest Disclosure
Authors declared that they do not have any conflict of interest.
Acknowledgements
Authors gratefully acknowledged the financial support provided by Department of Science and Technology, Government of India, New Delhi, in the form of project grant and thankful to CSIR-New Delhi for providing senior research fellowship.
References
- Newman DJ, Cragg GM (2007) Natural products as sources of new drugs over the last 25 years. J Nat Prod 70: 461-477.
- De Bary HA (1884) VergleichendeMorphologie und Biologie der Pilze, Mycetozoen und Bacterien. Verlag von Wilhelm Engelmann, Leipzig, Germany.
- Verma VC, Kharwar RN, Strobel GA (2009) Chemical and functional diversity of natural products from plant associated endophytes. Nat Prod Commun 4: 1511-1532.
- Tan RX, Zou WX (2001) Endophytes: a rich source of functional metabolites. Nat Prod Rep 18: 448-459.
- Chandra S (2012) Endophytic fungi: novel sources of anticancer lead molecules. ApplMicrobiolBiotechnol 95: 47-59.
- Demain AL (1999) Pharmaceutically active secondary metabolites of microorganisms. ApplMicrobiolBiotechnol 52: 455-463.
- Strobel GA (2003) Endophytes as sources of bioactive products. Microbes Infect 5: 535-544.
- Strobel G, Daisy B (2003) Bioprospecting for microbial endophytes and their natural products. MicrobiolMolBiol Rev67: 491-502.
- Schulz B, Wanke U, Draeger S, Aust HJ (1993) Endophytes from herbaceous plants and shrubs: effectiveness of surface sterilisation methods. Mycol Res 97: 1447-1450.
- Kajaria DK, Gangwar M, Kumar D, Sharma AK, Tilak R, et al. (2012) Evaluation of Antimicrobial Activity and Bronchodialator Effect of a Polyherbal Drug-Shrishadi. Asian Pac J Trop Biomed 2: 905-909.
- National Committee for Clinical Laboratory Standards (2004) Performance standards for antimicrobial susceptibility testing. Fourteenth Informational Supplement NCCLS documents M100-S14. NCCLS, Wayne, Penn., U S A.
- Gangwar M, Kumar D, Tilak R, Singh TD, Singh SK, et al. (2011) Qualitative phytochemical characterization and antibacterial evaluation of glandular hairs of Mallotusphilippinensis fruit extract. J Pharm Res 4: 4214-4216.
- Verma VC, Gond SK, Mishra A, Kumar A, Kharwar RN, et al. (2009) Endophyticactinomycetes from Azadirachtaindica A. Juss.: Isolation, diversity and anti-microbial activity. MicrobEcol 57: 749-756.
- Verma VC, Lobkovsky E, Gange AC, Singh SK, Prakash S (2011) Piperine production by endophyticPericonia sp. isolated from Piper longum L. J Antibiot 64: 427-431.
- Fiedler HP, Brunter C, Bull AT, Ward AC, Goodfellow M, et al. (2005) Marine actinomycetes as a source of novel secondary metabolites. Antonie Van Leeuwenhoek 87: 37-42.
- Tan RX, Zou WX (2001) Endophtes: A rich source of functional metabolites. Nat Prod Rep 18: 448-459.
- Gangwar M, Dalai A, Chaudhary A, Singh TD, Singh SK, et al. (2012) Study on activity of alcoholic extract of glands and hairs of fruits of Mallotusphilippinensis in murine cestodal infection model. Int J Pharm PharmceuSci 4: 643-645.
- Gangadevi V, MuthumaryJ (2008) Taxol, an anticancer drug produced by an endophytic fungus BartaliniarobillardoidesTassi, isolated from a medicinal plant Aeglemaemelos Correa ex Roxb. World J MicrobiolBiotechnol 24: 717-724.
- Balandrin MF, Kinghorn AD, Farnsworth NR (1993) Plant-derived natural products in drug discovery and development. In: Human Medicinal Agents from plants. Am ChemSoc, Washington, USA.
- Hormazabal E, Piontelli E (2009) Endophytic fungi from Chilean native gymnosperms: antimicrobial activity against human and phytopathogenic fungi. World MicrobiolBiotechnol 25: 813-819.
Copyright: © 2015 Gangawar M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.