Journal of Agricultural Science and Food Research
Open Access
ISSN: 2593-9173
ISSN: 2593-9173
Research Article - (0) Volume 6, Issue 6
Rock Phosphate is the cheapest and abundant Phosphatic fertilizer available but due to its sparse solubility it is not always agronomically effective. Combined application of rock phosphate with phosphate solubilizing microorganisms has emerged as a logical solution to this issue. In the present investigation, random soil samples were collected from sites in and around Vadodara to isolate rock phosphate solubilizing fungal strains. Thirty different fungal strains were chosen for rock phosphate solubilization in 5% senegal rock phosphate as a source of phosphorus in Pikovskayas’s medium. Out of these, three isolates exhibited maximum rock phosphate solubilization i.e., 92 ppm, 381 ppm and 297 ppm in seven days with a considerable decrease in pH. Bio-fertilizer activity of these isolates in combination with rock phosphate was also individually tested on Pennisetum glaucum (bajra) in pots under natural environmental conditions. Most of the biometric parameters were increased after this treatment. The results indicate positive effect of co-application of rock phosphate with phosphate solubilizing fungi on plant growth.
<Keywords: Biofertilizers,Fungi, Pot studies
Phosphorus (P) is one of the cardinal members of macronutrients required by plants. It rapidly gets immobilized when added as a soluble fertilizer [1]. Due to immobilization, P becomes less available for plant, calling for frequent reapplication which is costly and an environmental unfriendly solution [2]. Use of less expensive sources of phosphorous is advocated for sustainable agriculture approaches [3,4]. Presently, rock phosphate (RP) is being chiefly employed to sustain soil P level in available form for plants [5]. Poor solubility of RP is the major drawback in its use as a phosphatic fertilizer. Commonly physical and chemical efforts like decreasing particle size and partial acidulation, convert such rocks to more valuable product [6]. Reports have shown that availability of P in a soil can be enhanced by induced microbial solubilisation of phosphates [7,8]. Many bacteria and fungi are able to improve plant growth by solubilizing inorganic and organic phosphates in the soil [9,10]. Fungi have been reported to possess greater ability to solubilize RP than bacteria [11]. They are considered as one of the key groups of soil micro flora involved in P cycling. Among them, Penicillium and Aspergillusare two genera frequently used for phosphate solubilization [12]. Researchers have shown that RP application as a phosphate fertilizer along with the activity of soil microorganisms can be effective [13]. The combinatorial application of phosphate solubilizing microbes (PSM) and RP is an excellent bio-fertilizer and has become increasingly important against depletion of high-grade RP reserves [14]. In the present study, RP solubilizing fungi were isolated from rhizosphere soil of crop fields in and around Vadodara. The strains were tested for their capacity to solubilize Senegal RP which is used as a raw material for phosphoric acid production. Qualitative and quantitative estimation of phosphate solubilization activity of the fungal isolates was carried out in modified Pikovaskaya’s (PVK) medium. Isolates showing best inorganic phosphate solubilizing activity were evaluated in pot studies using Pennisetum glaucum (bajra) under natural environmental conditions.
Chemicals and culture media
Rock phosphate: Senegal RP having 32.2% P2O5 was used for the study. Detailed RP mineral content analysis was done in house quality control facility Table 1.
| Parameters | Senegal RP (%) | Parameters | Senegal RP (%) |
|---|---|---|---|
| %Moisture | 2.49 | %T-Na2O | 0.2 |
| % LOI* | 4 | %T-K2O | 0.05 |
| %Acid Soluble | 6.71 | %SO3 | 0.27 |
| % T#-Silica | 8.74 | %CO2 | 1.62 |
| %T-CaO | 45.39 | %MgO | 0.18 |
| %T-P2O5 | 32.23 | Chloride ppm | 75 |
| %Fluoride (F) | 3.12 | %Reactive SiO2 | -- |
| %Fe2O3 | 1.79 | %Al2O3 | 2.88 |
*Loss on ingnition, # Total
Table 1: Detailed RP mineral content analysis.
Potato dextrose agar (PDA) and broth (PDB): HI Media readymade PDA (Potatoes infusion 200 g/L, Dextrose 20 g/L, Agar 15 g/L Final pH (at 25°C) 5.6 ± 0.2) and PDB were used for primary isolation purpose.
Pikovaskaya’s medium (PVK): A modified PVK medium (37) containing Yeast extract 0.5 g/L, Dextrose 10 g/L, Ammonium sulphate 0.5 g/L, Rock phosphate 5 g/L (700 ppm of P), Potassium chloride 0.2 g/L, Magnesium sulphate 0.1 g/L, Manganese sulphate 0.0001 g/L, Ferrous sulphate 0.0001 g/L, Agar 15 g/L, bromophenol blue 2.4 g/L [15] was autoclaved and used for isolation of RP solubilizing fungus.
Isolation of microorganism
RP solubilizing fungi were isolated from rhizosphere soil of crop fields using the dilution plate method. Soil sample (10 g) was mixed with 90 mL sterile distilled water. It was vigorously shaken and left to stand for 5 min. Homogeneous soil solution was serially diluted using normal saline. Dilutions from 10-4, 10-5 and 10-6 were plated on PDA and incubated at 28 ± 2°C for 3 days. Isolated fungal strains were maintained on PDA slants until use. Phosphate solubilizing ability of the isolates was confirmed by incubating them on PVK medium incorporated with 5% Senegal RP at 28 ± 2°C for 7 days. Diameter of clearance zone was measured successively after 24 h, up to 7 days. The Solubilization Index (SI) is the ratio of total diameter i.e. clearance zone including fungal growth and the colony diameter. All the observations were recorded in triplicate.
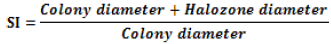
RP Solubilisation in liquid culture
Microorganisms were tested in liquid media to assess their capability of releasing P from insoluble sources. Modified PVK broth (100 mL with 5% Senegal RP) was distributed into 500 mL Erlenmeyer flasks and the pH was adjusted to 7.0. After sterilisation and cooling, 1mL mycelia suspensions were used to inoculate flasks in triplicate. Non-inoculated flasks supplemented with RP served as controls. Incubation was made at 28 ± 2°C, 150 rpm for 30 days with intermittent sample withdrawal (centrifuged at 17250 g for 10 min) for P solubilization, pH measurement and organic acid analysis on 7th, 14th and 21st day. The quantitative estimation of solubilized P was done by the Vanadomolybdophosphoric yellow color method [16].
Determination of organic acid in the culture medium
Organic acid production was estimated by High Performance Liquid Chromatography (HPLC) analysis. HPLC operating parameters for the analysis of organic acids were: column: Waters, symmetry shield C18, 250 x 4.6 mm, 5 μm; detector: UV 210 nm; mobile phase: water containing 0.2% w/w phosphoric acid in water; flow rate: 0.7 ml/min; column temperature: 30°C; run time: 25 min; standard used: 100 ppm of each organic acid in water.
Organic Acid content (ppm) was calculated by following formula.
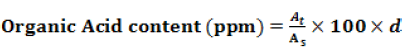
Where At, As, and d, are area of sample, area of standard, and dilution factor of sample
Pot studies
Spore suspension for inoculation: The three RP solubilizing strains were mass cultured aseptically in 90 mm diameter Petri plates each containing 15 mL of autoclaved PDA. The plates were incubated at 28 ± 2°C for 10 days. On the tenth day spore suspensions from the fungal inoculum were prepared by flooding the surface of the agar with 10 mL sterile distilled water (with 0.01% Triton X-100) and the culture surface gently scraped to dislodge the spores. The spore suspension was transferred separately to 500 mL flask containing 400 mL sterile distilled water. Flasks were shaken for 2 minutes to ensure that the spores were properly mixed. The spore count was determined using Haemocytometer with a formula mentioned below, so that the final counts were determined as 1 × 107spores/mL:
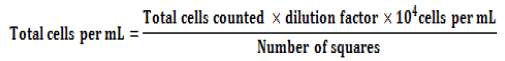
Seed germination assay: Bajra seeds were surface sterilized with 0.5% sodium hypochlorite, and rinsed several times with sterile distilled water. These seeds were treated with fungal spore suspension. Ten seeds were then placed at equal distance on the aseptically moist blotter paper of 8.5 cm diameter in pre-sterilized borosil glass Petri dishes of 10 cm diameter. This Petri dish was incubated at 28 ± 2°C for 6 days. After incubation period, percent seed germination, shoot and root length of each seeds was recorded.
Pot experiment: A greenhouse experiment with randomized complete block design in factorial form with three replications of each treatment (T2-T7) and one control (T1) was conducted. After germination assay the seedlings were transplanted into sterilized plastic pots (12 × 6.45 cm) containing 1.5 kg nursery soil. All treated pots were inoculated with 5 mL of fungal spore suspension at a final concentration of 1 × 107 cell/mL. Treatment pots T5-T7 were supplemented with 4 g/kg senegal RP. All the pots were kept in natural condition for 21 days. After completion of trial the plant were washed thoroughly with water and several parameters like length of roots and shoots, number and length of leaves, dry weight of plants etc. were recorded for comparative evaluation in different combinations.
Soil analysis: Analysis of mineral elements was done by sampling soil before the experiments and after harvesting the plants. Soil samples were air dried and sieved (2 mm screen) and analyzed for the following parameters: pH (1:2 water extraction), total nitrogen by Kjeldahl method [17], organic carbon by Wakley and Black method [18], available phosphorus (P2O5 Kg/Ac) by Olsen’s method [19], available potash in (K2OKg /Ac) by ammonium acetate method [20], Electrical conductivity (Desisymen / metre (EC 1:2), and micronutirents like Sulfur, Zn , Fe , Mn , Cu in ppm by Diethylene tetramine penta acetic acid (DTPA) extraction method using atomic absorption spectrophotometer [21].
Identification of fungal strains
Identification of fungal isolates was done by observing colony characteristics on PDA plates. On the basis of growth pattern the isolates were identified as Aspergillus and Penicillium spp. This was confirmed by microscopic analysis of colony using lacto phenol blue stain. Aspergillus gave black dense felt like mycelial growth on front side of PDA plate with dirty white color on back side Figure 1. Microscopic analysis showed long conidiophores with terminal smooth sac like structure. Round black conidia were arranged in chains on this sac. Penicillium spp. showed grey and yellow color colonies on front side of PDA plate while orange on back side Figure 1. Microscopically, branched conidiophores and phialides were observed giving brush-like appearance. Conidia were globular, greenish and smooth.
Determination of RP Solubilization in solid medium
Out of all fungi isolated from the soil only three fungi showed significant zone of phosphate solubilisation Figure 2. A clear halo zone was formed around the colonies after 7 days of incubation on blue colored PVK medium incorporated with 5% senegal RP indicating phosphate-solubilizing ability of the fungal isolates. Maximum SI (3.5) was shown by Aspergillus followed by Penicillium sp.1 (3.3) and Penicillium sp.2 (3).
Mineral P solubilization and its correlation with acid production
In liquid media, all the three isolates gave fine globular growth of mycelium and decolorized the brown color broth Figure 3. Quantitative estimation of phosphate solubilization by these isolates was studied for 21 days. It was observed that solubilization started from fourth day, reached maximum on seventh day. RP solubilization was accompanied by decrease in pH (initial pH 7) in case of all isolates. Maximum drop in pH for all treatments was recorded on 7th day Table 2. Among all, Aspergillus solubilized maximum amount of RP (392 ppm). During experiments it produced significant amount of citric (237 ppm), gluconic (156 ppm) acid and moderate amount of oxalic acid (80 ppm). Additionally, some other organic acids, viz. succinic (30 ppm) glycolic (15 ppm) and malic acid (32 ppm) were also produced. Penicillium sp. 1 and sp. 2 mineralized 381 ppm and 297 ppm of RP respectively. Gluconic (185 ppm) and glycolic (158 ppm) acids were detected in higher amount in treatments with Penicillium sp. 1, which contributed most to RP solubilization. In case of Penicillium sp. 2, except malic acid (122 ppm), all other organic acids like citric, gluconic, glycolic, succinic and oxalic acids were in low concentrations which resulted in moderate RP dissolution.
| Aspergillus | Penicilliumsp.1 | Penicilliumsp.2 | |
|---|---|---|---|
| Soluble P (ppm) | 392 | 381 | 297 |
| pH | 3.2 | 3.3 | 3.8 |
| Organic Acids (ppm) | |||
| Citric Acid | 237 | 16 | 5 |
| Gluconic Acid | 156 | 185 | 24 |
| Glycolic Acid | 15 | 158 | 33 |
| Oxalic Acid | 80 | 22 | 7 |
| Malic Acid | 32 | 14 | 122 |
| Succinic Acid | 30 | 33 | 2 |
Table 2: RP solubilization, pH and organic acid profile of fungal isolates.
Effect of fungal isolates on plant growth
Seed germination: It was observed that treated and control groups showed almost equal number of seed germination. Length of radical and plumule in all the seeds were comparable Figure 4. At the sampling stage after seven days of germination, root length, shoot length and weight of treated groups were found to be more than controlled ones.
Plant growth in pots: As the plants continued to grow significant variations in the growth parameters of different fungi inoculated bajra plants were observed Table 3. Penicillium sp. 1 and Aspergillus exhibited a remarkable increase in plant growth as compared to the controls. Penicilliumsp. 1 when added to soil in combination with rock phosphate showed 61% and 47% increase in root and shoot length respectively. Similarly, Aspergillus with rock phosphate gave 56% and 50% increase in root and shoot length. However, Penicillium sp. 2 induced moderate plant growth of 38% and 28% in root and shoot length. Both the Penicillium sp. 1 and Aspergillus showed 50% augmentation in length and number of bajra leaves. Penicillium sp. 2, however, gave 25% and 30% increase in length and number of leaves respectively. Penicillium sp. 1 and Aspergillus isolates increased weight of plant in a range of 53-56%, while treatment with Penicillium sp. 2 gave 32-34% increase in plant weight.
Soil analysis: Most of the biometric parameters of soil were enhanced after addition of fungal isolates Table 3. Fungal treatment increased the available P of soil up to 50% as compared to control. A moderate increase of available K in a range of 7-17% was also observed during the study. It was found that inoculation of fungal isolates reduced the soil pH from 8.2 to 7.0. It was also observed that, as pH shifted towards neutrality the organic carbon and nitrogen content of soil was increased by 50% in treated groups. Along with this, lowering in pH of treated sets was also accompanied by increase in conductivity in a range of 28-43%. The level of different micro-nutrients was also found to be enhanced due to microbial treatment.
In agreement with several published reports, the present study confirms high phosphate solubilizing potential of common soil fungal strains Aspergillus and Penicillium [22-24]. During the study these isolates released 45-50% RP in seven days, which is in corroboration with previous findings [3,25,26]. Here, RP solubilization was accompanied by lowering of pH, suggesting production of organic acids by fungi, which were confirmed by HPLC analysis of samples. Aspergillus, during experiments produced generous amount of citric acid, which is considered responsible for maximum RP solubilization. Earlier reports on correlation between citric acid production and RP solubilisation by Aspergillus support these observations [25,27]. Apart from citric, Aspergillus, during experiments also released fair amount of oxalic acid, which is already known to play important role in RP solubilization [28]. Here, in case of Penicillium, gluconic, glycolic and malic acids are responsible for RP solubilization, which is consistent with published results [29-31]. In addition to phosphate solubilization in laboratory conditions, plant growth promotional ability of Aspergillus and Penicillium in pot and field experiments have also been reported by several authors [10,32,33]. Similar effects were observed during the present study when these isolates were applied alone or in combination with RP powder. This study revealed that amendment of RP in soil along with phosphate solubilizing fungus can increase plant growth, which is in corroboration with known findings [31,34,35]. Additionally these isolates induced various physical, biological, and chemical soil properties as well. Neutrality of soil was achieved after treatment which in turn enhances its electrical conductivity. Overall nutritive value of soil also increases after this treatment and these results are in line with available results [36]. These observations support that co-application of RP solubilizing fungal isolates with RP, has a positive effect on plant growth [37]. It may provide an environmentally desirable alternative to phosphorous fertilization in the light of sustainable agriculture approaches.
The author acknowledges Gujarat State Fertilizers and Chemicals Ltd. for financial support. We would also like to extend regards to Dr. N. Mishra, (SR.VP - HR, IR, T&R, QC & SERVICES), Mr. A. S. Sikdar (VP – PU, R&D, QC and MR) and Dr. D.H. Garg (OSD- Research) for supporting this research work.