Journal of Agricultural Science and Food Research
Open Access
ISSN: 2593-9173
ISSN: 2593-9173
Research Article - (2011) Volume 2, Issue 2
Soil samples from various agro climatic zones of Andhra Pradesh, India were collected and screened for the isolation of effective microbes during 2009-10. Based on the efficacy and in vitro compatibility, nine microbial strains (Bacillus spp, Streptomyces spp, Azotobaacter spp and Frauteria spp) were identified and christened (VBT 01 to VBT 09). Each strain was evaluated for its plant growth promoting efficacy (PGP) in black gram (Vigna mungo (L.) Hepper) and its antagonistic activities against select pathogens. Pot culture studies recorded high percentage of seed germination (94.7%) when the black gram seeds were coated with VBT 09 as compared to untreated control (83.2%). Maximum radical length of 8.0 cm was recorded in VBT 09 as compared to control (0.53cm). PGP traits of all the strains such as Hydrogen cyanide (HCN), ammonia production and hydrolytic enzymes like cellulase, pectinase, protease and amylase were recorded at various levels. The positive production of HCN was recorded in VBT 05. Antagonistic activity was recorded against Macrophomina phaseolina (VBT 03), Sclerotium rolfsii (VBT 07), Fusarium oxysporum (VBT 05) and Rhizoctonia solani (VBT 04). Mycoparasitic activity of VBT 02 strain was significantly high (53.8% inhibition over control) against sclerotial bodies of Sclerotium rolfsii. However, none of the strains exhibited sclerotial parasitism against Rhizoctonia solani. Pot culture assay to study the consortia effect at different proportions recorded significant increase in shoot length (11.2cm) as compared to chemical fertilizer (9.77cm) and control (8.51cm).The root volume and shoot dry mass was 0.5cm3 and 0.15cm3 as compared to control 0.17cm3 and 0.09cm3 respectively. The total dry mass was higher in consortia treated pots (0.23g) than the pots treated with chemical fertilizer (0.2g). The consortium is named as Omega. An on-farm trial is being carried out to evaluate its efficacy as soil conditioner, plant growth promoter and disease resistance capabilities in paddy and sugarcane.
<Keywords: Consortia; Plant growth promotion; Antagonism and mycoparasitism; Effective microbes; Streptomyces sp; Bacillus sp; Azotobacter sp; Frauteria sp; Rhizobacteria
Plant growth promoting microorganisms (PGPM) are heterogeneous in nature comprising bacteria, fungi and actinomycetes that survive in and around the root rhizosphere. PGPM enhance the plant growth and yield either directly or indirectly [1]. The direct plant growth promotion involves in the solubilization or mobilization of important nutrients (phosphorous, potash, zinc, sulphur, and iron) or fixing atmospheric nitrogen for the uptake of plants. They are also known to produce various plant growth promoting hormones like indole acetic acid, gibberlic acid, cytokinins and ethylene [2]. PGPM also indirectly reduces the deleterious effect of phytopathogens.
The modes of action of PGPM though not completely explored, the possible reasons could be: 1. Production of plant growth regulators, 2. Symbiotic and asymbiotic N2 fixation [3], 3. Antagonistic activity to phytopathogens by the production of siderophore [4], antibiotics [5] and HCN [6], 4. Solubilization of mineral phosphates and other nutrients [7], 5. Substrate competition, 6. Chitinase production, 7.Cellulase, pectinase, protease & starch hydrolysis and 8. Sclerotial and mycoparasitization. In addition to these traits, an effective PGPM should be a rhizosperic competent, able to cope with the biotic and abiotic stresses and colonize in the rhizospere [8].
Bacillus spp, Pseudomonas spp, Azospirillum spp, Rhizobium spp, Azotobacter spp, Klebsiella spp and Serratia spp are proven to enhance the plant growth [9]. Pseudomonas fluorescens and Bacillus subtilis were indeed brought under pesticide act in India due to their better performance in the field. However, the inconsistency and variability in the performance of PGPM is a major hindrance factor that affects their efficacy. Factors like adverse agro-climatic conditions that include climate, weather parameters and soil characteristics are reported to be some of the important hindrance factors. It is always an elusive task for a single microbial inoculant to perform consistently under stress. More importantly, the results obtained in vitro cannot be always dependably reproduced under field conditions [10].
Numerous recent studies showed promising trends in PGPM research [11-13]. One such technology is mixed inoculant (microbial consortia) that interacts synergistically with each other, enhances the plant growth and subsequently protects them from phytopathogens. Therefore, in this paper, Varsha Bioscience and Technology India Pvt Ltd in association with Telengana University evaluated nine effective PGPM in terms of compatibility, nutrient convertibility/mobility and antagonistic activity against phytopathogens. Based on the results obtained, a wettable powder formulation was made successfully comprising all the nine strains with different proportions. The product was christened as Omega and the results obtained from the study are provided in This article.
Microbial cultures
Soil samples were collected from the rhizosphere of crops viz., cotton, chilli, paddy, banana, sugarcane and chickpea across the state of Andhra Pradesh. Collected samples were brought to the laboratory as per the standard protocol. Among the isolated microbial population, 14 strains were basically identified as effective microbes. Based on the efficacy and in vitro compatibility, nine effective microbes were selected and named as VBT 01 to VBT 09. The nine strains were basically identified and grouped under the genus Bacillus spp, Streptomyces spp, Azotobacter spp and Frauteria spp.
In vitro seed germination assay
Seeds of Sorghum (cv CSV 15) were surface sterilized with 0.1% HgCl2 for 3 min and washed 5 times with sterile distilled water. Subsequently, seeds were soaked for 1 min in 70% ethanol and washed thrice with sterile distilled water. Washed seeds were immersed individually in 48-h old culture of VBT 01 to VBT 09 for 5 min, transferred on the water agar plates (agar- 1.5%) @ 9 seeds/plate and incubated for 4 days at 30°C. Seeds soaked in sterile media were kept as control. The percent seed germination and radicle length were recorded on day-4.
Detection of PGP traits
All the nine strains were evaluated individually to identify the presence of PGP traits using the methods for HCN [14], Ammonia and protease [15], Cellulase [16], Pectinase [17] and Amylase [18] production.
In vitro antagonistic activity against phytopathogens
In vitro studies were carried out to evaluate the antagonistic activity of all the nine strains against selected phytopathogens viz., Sclerotium rolfsii, Rhizoctonia solani, Fusarium oxysporum and Macrophomina phaseolina by dual culture method [19]. The percent inhibition over the control was measured by the formulae:
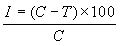
where,
I = Inhibition % of mycelial growth (growth reduction over control)
C= Radial growth of pathogen without antagonist
T= Radial growth of pathogen with antagonist
Mycoparasitism over sclerotial bodies of Sclerotium rolfsii and Rhizoctonia solani
The antagonistic effect of Omega strains on sclerotial germination of S. rolfsii and R. solani were determined by adopting the method [20] with slight modifications. Ten-day old sclerotia of the pathogen that are produced on PDA are collected and surface sterilized in 2.5% sodium hypochlorite solution. The Omega isolates were multiplied in Erlenmeyer flasks by adding a loop ful of actively growing cultures in 50 ml of Tryptic Soy Broth (10% TSB) and incubating for 24-h on a rotary shaker at 175 rpm at room temperature (26±2°C). Five sclerotia of each pathogen were later inoculated in flasks of each bacterial isolate and were placed for a period of 24-h on a rotary shaker at 175 rpm at room temperature. The sclerotia are then removed and subsequently placed onto PDA plates and incubated for 72-h at 26°C and the germination and radial growth was measured. Controls were run separately for each bacterial isolate by replacing inoculants with 1ml of sterile distilled water. Each experiment considering nine isolates were run in five replications and observations on percent sclerotial germination were recorded. The radial growth of mycelium from sclerotia is recorded and percent inhibition of rate of mycelial growth from sclerotia was determined by using the following formula.
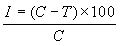
where, I= inhibition of mycelial growth, C= mycelial growth of pathogen in the control plate (mm) and T= growth of pathogen in treatment (mm).
Evaluation of plant growth promotion by short duration plant test
Soil was autoclaved at 121°C for 1 hour for 3 consecutive days to kill the existing microbes in the soil. Each pot was filled with 250 g of sterile soil. Seeds of the test crop, Vigna mungo (Black gram cv T9) were surface sterilized with 0.1% HgCl2 for 3 min and washed 5 times with sterile distilled water. Subsequently, seeds were soaked for 1 min in 70% ethanol, washed thrice with sterile distilled water and kept over the sterile blotting paper to remove the excess moisture. The seeds were treated uniformly with 100mg of Omega per 100g of seed, air dried and sown. Twelve replicates were maintained for each treatment containing 3 seeds per pot. Simultaneously, a positive control was maintained by treating the soil with recommended doses of chemical fertilizers (20N:30P:30K). Pots filled with soil without Omega and chemical fertilizers were sown and kept as negative control. The percentage of seed germination and radical length were recorded at 10 days and 30- days after sowing, respectively.
The values presented are the means of two experiments each with six replicates performed at different occasions. Data obtained from all the experiments were subjected to two-way analysis of variance (ANOVA). Mean values between treatments were compared with Fisher's Least Significant Difference (L.S.D) test (P<0.05).
In vitro seed germination assay
Significant germination (%) and radicle elongation (cm) was recorded when the seeds were treated with VBT 09 (94.7% & 8.0 cm), VBT 04 (90.2% & 4.23 cm) and VBT 03 (89.5% & 2.96 cm) as compared to untreated control (83.2% & 0.53 cm) respectively.
Plant growth promoting traits of Omega strains
Results on PGP traits of Omega strains are depicted in Table 2. Among nine strains, VBT 05 was the only strain found to be a HCN producer (Figure 1). Ammonia production was observed in all the strains. However, the degree of production ranged from moderate (VBT 01, VBT 02, VBT 05 and VBT 08) to weak (VBT 03, VBT 04, VBT 06, VBT 07 and VBT 09) as compared to control. All the strains showed positive production of cellulase and pectinase in which VBT 01, VBT 02 and VBT 04 were found to be the strong producers. VBT 02 and VBT 03 exhibited strong hydrolytic action against casein protein and starch (Figure 2).
| Strains | % germination | Radical length in cms |
|---|---|---|
| Control | 83.2 | 0.53d (±0.05) |
| VBT01 | 84.3 | 0.58d (±0.01) |
| VBT02 | 87.1 | 1.66d (±0.05) |
| VBT03 | 89.5 | 2.96c (±0.08) |
| VBT04 | 90.2 | 4.23b(±0.5) |
| VBT05 | 85.0 | 1.0d (±0.08) |
| VBT06 | 88.1 | 3.0c (±0.12) |
| VBT07 | 87.4 | 1.7d (±0.13) |
| VBT08 | 83.6 | 0.53d (±0.04) |
| VBT09 | 94.7 | 8.0a (±1.35) |
| LSD | 1.076 | |
| CV% | 91.53 |
Values superscripted by same alphabet are not significantly different according to Fisher’s Least Significance Difference test (P<0.05). Values in the brackets are standard errors (SE).
Table 1: Effect on seed germination and radicle length by Omega strains.
| Strains | HCN production | Ammonium production | Hydrolysis of complex source | |||
|---|---|---|---|---|---|---|
| Cellulase | Pectinase | Protease | Amylase | |||
| VBT01 | - | ++ | +++ | +++ | +++ | ++ |
| VBT02 | - | ++ | +++ | +++ | +++ | +++ |
| VBT03 | - | + | ++ | + | +++ | +++ |
| VBT04 | - | + | +++ | +++ | +++ | ++ |
| VBT05 | +++ | ++ | +++ | ++ | +++ | + |
| VBT06 | - | + | ++ | +++ | ++ | - |
| VBT07 | - | + | ++ | + | + | ++ |
| VBT08 | - | ++ | + | + | + | - |
| VBT09 | - | + | + | + | - | - |
+++ strong, ++ medium, + weak, - negative producer.
Table 2: PGP traits of Omega strains.
In vitro antagonistic activity against phytopathogens
Antagonistic activity of Omega strains exhibited diversified percent inhibition against select phytopathogens (Table 3). VBT 09 strain recorded maximum inhibition against M. phaseolina (45.59%) followed by VBT 03 (45.21%). Against S. rolfsii, VBT 07 showed 55.04% inhibition followed by VBT 06 with 43.18%. F. oxysporum growth was effectively minimized by strain VBT 05 (49.84%) followed by VBT 09 (49.04%). In case of R. solani, 84.94% inhibition was recorded by VBT 04 strain. VBT 01 and VBT 03 were the next best strains by showing 65.72% and 54.45% inhibition, respectively.
| Strain | % Inhibition over control | |||
|---|---|---|---|---|
| M. phaseolina | S. rolfsii | F. oxysporum | R. solani | |
| VBT01 | 38.93b (±1.79) | 0.0 | 43.33c(±0.16) | 65.72b(±0.19) |
| VBT02 | 39.19b (±1.80) | 8.45g (±0.35) | 45.57b(±0.52) | 42.8d(±0.20) |
| VBT03 | 45.21a (±2.08) | 21.97e(±0.56) | 48.92a(±0.45) | 54.45c(±0.56) |
| VBT04 | 8.45c (±0.38) | 33.35c(±0.67) | 45.47b(±0.54) | 84.94a(±0.43) |
| VBT05 | 0.0 | 17.32f(±0.40) | 49.84a(±0.47) | 41.09e(±0.46) |
| VBT06 | 38.93b(±1.79) | 43.18b(±0.81) | 43.18c(±0.38) | 33.72f(±0.45) |
| VBT07 | 39.71b (±1.83) | 55.04a(±0.33) | 38.29d(±0.35) | 0.0 |
| VBT08 | 0.0 | 28.31d(±0.27) | 43.91c(±0.29) | 0.0 |
| VBT09 | 45.59a (±2.10) | 0.0 | 49.04a(±0.39) | 0.0 |
| LSD | 2.17 | 1.0123 | 0.983 | 0.749 |
| CV% | 66.81 | 78.65 | 7.315 | 82.13 |
Values superscripted by same alphabet are not significantly different according to Fisher’s Least Significance Difference test (P<0.05). Values in the brackets are standard errors.
Table 3: Antagonistic activity of OMEGA strains against fungal phytopathogens.
Mycoparasitization activity on sclerotial bodies by OMEGA strains
Maximum sclerotial parasitisation was recorded in VBT 08 (54.4%) followed by VBT 02 (53.8%) strains against S. rolfsii as compared to control (Table 4). About 43.8% to 45.5% parasitisation was recorded in VBT 04 to VBT 06 strains. The parasitism was recorded least in VBT 09 (5.5%) and nil in VBT 01. Interestingly, none of the strains recorded sclerotial parasitisation against R.solani (Table 4).
| Strains | % Inhibition over control | |
|---|---|---|
| Sclerotium rolfsii | Rhizoctonia solani | |
| VBT01 | 0.0 | 0.0 |
| VBT02 | 53.8b(±0.41) | 0.0 |
| VBT03 | 28.2e(±0.29) | 0.0 |
| VBT04 | 45.5c(±0.35) | 0.0 |
| VBT05 | 43.8d(±0.24) | 0.0 |
| VBT06 | 45.5c(±0.36) | 0.0 |
| VBT07 | 45c(±0.43) | 0.0 |
| VBT08 | 54.4a(±0.42) | 0.0 |
| VBT09 | 5.5f(±0.23) | 0.0 |
| LSD | 0.713 | 0.0 |
| CV% | 54.78 | 0.0 |
Values superscripted by same alphabet are not significantly different according to Fisher’s Least Significance Difference test (P<0.05). Values in the brackets are standard errors.
Table 4: Sclerotial parasitisation by Omega strains.
| Treatments | Shoot length in cm | Root volume in cm3 | Shoot dry mass in g | Root dry mass in g | Total dry mass in g |
| Control | 8.51c(±0.22) | 0.17b(±0.01) | 0.09b(±0.01) | 0.04c(±0.0085) | 0.14c(±0.011) |
| Chemical | 9.77b(±0.19) | 0.39a(±0.01) | 0.13a(±0.01) | 0.06b(±0.0047) | 0.2b(±0.0081) |
| OMEGA | 11.21a(±0.32) | 0.50a(±0.09) | 0.15a(±0.1) | 0.07a(±0.0047) | 0.23a(±0.0062) |
| LSD | 0.70 | 0.14 | 0.028 | 0.0091 | 0.0099 |
| CV% | 12.59 | 48.93 | 29.11 | 34.59 | 21.93 |
Values superscripted by same alphabet are not significantly different according to Fisher’s Least Significance Difference test (P<0.05). Values in the brackets are standard errors.
Table 5: Plant growth promotion in black gram by Omega strains (30DAS).
Plant growth promotion in blackgram (Vigna mungo (L.) Hepper)
In the pot culture assay, seeds coated with Omega recorded significant increase in shoot length, root and total dry mass (11.21 cm, 0.07 g and 0.23g) as compared to fertilizer treatment (9.77 cm, 0.06 g and 0.23 g) and untreated control (8.51 cm, 0.04 g and 0.14 g) respectively. However, the results on root volume and shoot dry mass in omega treated pots are on par with pots treated with inorganic fertilizers (Table 5). Visual observation indicated better fibrous root formation in omega treatment as compared to inorganic fertilizer treatments though the root length was marginally high in fertilizer treated cups compared to control (Figure 3).
The role of effective microbes for plant growth, development and yield is well documented [21,22]. The scientific understanding of the plant growth promotion is the production of plant hormones and other growth related activities like providing nutrients [23], release of volatile organic compounds in the rhizopshere, and suppression of plant disease causing pathogens [24,25] and insect pests like whitefly [26] either directly or indirectly.
More importantly, consortia of microbes in the rhizosphere is reported to act synergistically by stimulating each other through physical and/or biochemical process and provides essential nutrients to plants and protect them from disease causing microbes [27]. For example, plant growth is significantly increased when Azospirillum is used with phosphate solubilising bacteria [28]. In China, 18 commercial PGPR strain mixtures are sold, most of which are derived from the spore-forming genus Bacillus [21]. Therefore, in the present study, nine microbes (VBT 01 to VBT 09) are short listed and evaluated for their capabilities on plant growth, antagonistic activities against plant pathogens and possible ability to convert plant residues into organic carbon.
Seed germination
The strain, VBT 09 proved to enhance the germination of black gram (94.7%) with excellent radical elongation (8cm) as compared to other strains and untreated control. Though VBT 09 reported to produce protease, amylase, cellulase, pectinase and ammonia with limited capabilities (Table 2), the germination and radical length in VBT 09 treated seeds are better than the seeds treated with other strains. This could be due to the ability of the culture to produce hormones like Indole Acetic Acid [29].
Root and shoot growth
Consortia containing all the strains (Omega) has increased the shoot length, root dry mass and the total dry mass as compared to treatment with chemical fertilizers and untreated control. Though the root volume is statistically on par in treatment with chemical fertilizer and Omega, more fibrous roots are noticed in treatment using Omega as compared to treatment with chemical fertilizers and untreated control (Table 5). This kind of increase in overall root growth, greater production of root hairs, and enhanced root surface area is reported in PGPR like Azospirillum [10]. The overall shoot and root growth is also attributed due to the production of hormones like IAA, gibberellins, and volatile organic compounds [30-34].
Antagonistic activities
Consortia of microbes contained in Omega proved to be effective in suppressing the pathogenic fungi like M. phaseolina, S. rolfsii, F. oxysporum and R. solani (Table 3). The antagonistic effect of Omega against these pathogens could be due to the secretion of mixtures of antagonists like enzymes, phenolics, antifungal metabolites, signal compounds and other determinants in response to plant pathogens like fungi, bacteria, viruses, nematodes etc., [35-40] or antibiotics like bacilysin, iturin-like lipopeptides, diacetylphloroglucinol and pyrrolnitrin, HCN, phenazine-1-carboxylate [41]. Though laboratory assay indicated the above stated mechanisms, multi-location onfarm evaluations are required to confirm that Omega also works with induced systemic resistance mechanism as described by Van Loon et al. [42,43]. This merits attention since VBT 05 reported to produce HCN (Table 2).
Sclerotia are vegetative structures that play a major role in survival of fungi under adverse conditions. The sclerotia of the plant pathogen Rhizoctonia solani exude liquid brown droplets that have bioactivity and toxicity against microorganisms and plant species [44]. Their metabolic compositions of exudates are complex mixtures composed of phenolics (17.40%), carboxylic acids (12.79%), carbohydrates (6.08%), fatty acids (3.78%), and amino acids (3.47%). The presence of such metabolites contributed to their antimicrobial and phytotoxic activities. This could be one possible reason why all the strains of Omega did not exhibit sclerotial parasitisation activity on sclerotium of R. solani.
Soil fertility
The production of various hydrolytic enzymes (amylase, pectinase, protease and cellulose) by microbes could play an important role in soil fertility as they hydrolyze complex polysaccharides, proteins and urea into simpler form and add to soil again [45]. The cellulolytic activities of Omega strains indicated that it contains strains with strong capabilities to produce enzymes like cellulose, pectinase, protease and amylase (Table 2). Further, the level of ammonium production is from weak to medium. This could be the reason for the significant growth of shoot and root in Omega treated black gram as compared to control and fertilizer treatment (Figure 3 & Table 5).
In summary, Omega with nine effective microbes proved to be beneficial to black gram as efficient PGPR with good soil conditioning capabilities. It could also protect the plants from select soil borne fungal pathogens like M. phaseolina, S. rolfsii, F. oxysporum and R. solani. Further studies are necessary to explore the possible mechanism of plant growth promotion, nutrient mobilization and bio control activity. More importantly, on-farm field efficacy studies are essential against crops other than black gram. Therefore, field evaluation of Omega on paddy and sugarcane as multi-location study is currently being carried out at four locations in India.