Rheumatology: Current Research
Open Access
ISSN: 2161-1149 (Printed)
ISSN: 2161-1149 (Printed)
Research Article - (2021)Volume 11, Issue 5
Background: Whether the safety and efficacy of Januskinase Inhibitors (JAKis) in daily routine treatment of Rheumatoid Arthritis (RA) match the respective clinical trial results is of crucial clinical interest, however, growing, but still limited corresponding evidence is available.
Patients and methods: Data, including adverse events, disease activity scores, patient-related outcomes, and response as well as persistence rates, of all RA patients receiving JAKi treatment registered in the BioReg database were retrieved. The results were described according to those of pivotal clinical trials of JAKis. Analyses were performed using descriptive statistics, such as mean value comparisons, and by drawing patient trajectories.
Results: One-hundred-and twenty-two patients (mean age 64 years; 83.9% female, 60.5% Rheumatoid Factor (RF) positive; n=74 receiving Baricitinib and n=48 receiving Tofacitinib) were included from 2017 on. Significant differences occurred in the initial disease activity (mean Disease Activity Score including 28-joint-count (DAS28) 6.5 versus 3.8) between the trials and real-world data. In the registry, an insufficient response was observed in 24 (32.4%) and 21 (43.8%) patients, adverse events occurred in 16 (21.6%), and 12 (25.0%); the mean duration of treatment was 1.34 yrs. and 1.5 years. in patients receiving Baricitinib and Tofacitinib, respectively. The response rates were like those of the clinical trials, whereas adverse events were less frequently reported in the registry and no safety signals occurred. All scores applied showed a positive course and the results of mean value analysis and patient trajectories demonstrated the benefits of JAKis; the health assessment questionnaire values remained relatively stable, in contrast to the trial results.
Conclusion: Real World data for JAKis comply to a high extent with clinical trial results. This may contribute to an increased confidence in the therapeutic status of these drugs.
Rheumatoid Arthritis (RA); Januskinase Inhibitors (JAKis); Baricitinib; Clinical trial results
Rheumatoid Arthritis (RA), the most common chronic inflammatory disease, is a progressive, systemic autoimmune disease characterized by inflammation of the synovial membrane. This inflammation, aside from pain, stiffness, swelling, and loss of function, may also cause bone erosion, ultimately leading to irreversible joint destruction and disability [1]. Other systemic symptoms of RA include fatigue, anemia, and osteoporosis, and RA patient’s the overall risk to develop, example, cardiovascular diseases and infection is considerably increased [2,3]. Over the last decades, major advances in RA treatment have been achieved [4], due to the development of new biologic agents Biological Disease-Modifying Drugs (bDMARDS) with improved efficacy in an additional number of patients compared to conventional treatments, Conventional Synthetic DMARDs (csDMARDs), resulting in the change of treatment guidelines [5]. However, despite all advances, a considerable proportion of patients fail to achieve an acceptable clinical response or drug tolerance to the available treatments [6], including biologic compounds targeting Tumor Necrosis Factor-alpha (TNF-α), Interleukin-6 receptor (IL-6r), Interleukin 1 (IL-1), the co-stimulation pathway, or B-cells [6,7].
Meeting the obvious medical need for further effective treatments, a different therapeutic approach, targeting intracellular activation pathways, particularly Janus Kinases (JAKs), has been applied. JAK-Inhibitors (JAKis) have been shown to be more efficacious than methotrexate (MTX) and/or anti-TNF-a agents in clinical trials; providing an alternative for patients with an inadequate clinical response to biologic or non-biologic Disease Modifying Anti Rheumatic Drugs (DMARDs) [8]. Studies with the first two JAKis, Tofacitinib (Tofa) and Baricitinib (Bari) reimbursed in Austria since October 2017, and Baricitinib (Bari), reimbursed in Austria since August 2018, have demonstrated efficacy in several RA patient groups either as monotherapy or in combination with MTX and Adalimumab, as a comparator [9]. Those studies lead to a licensing of Bari and tofa as monotherapy or in combination with MTX in patients with moderate-to-severe RA not adequately responding to one or more DMARDs [10,11]. At the time point this evaluation was carried out, almost no Real-World Evidence (RWE) for the application of both drugs was available, which could allow a better understanding of the JAKis treatment impact in more diverse patient populations. Meanwhile, a feast of papers from different countries and cultures dealing with this topic have been published, providing evidence important for the clinically working rheumatologist, whether the risk/benefit ration of Januskinase Inhibitors (JAKis) in daily routine treatment of Rheumatoid Arthritis (RA) can be regarded reasonable, and which patient groups could expect benefits from JAKI treatment. However, most of the papers deal with different questions, in part comparing JAKis with biological, or giving information about treatment strategies; similarly to our investigations, the sample sizes are relatively small, even from big countries like Germany and Italy [12-16].
The objectives of the present evaluation of these registry data were to evaluate the safety and efficacy of JAKis in routine treatment of RA in real-life as well as to investigate whether real-life data match the respective clinical trial results to increase the knowledge about the position of JAKis in routine therapy of RA and possibly create a hypothesis for future studies. This paper presents the respective results from Austria, a relatively small country with a highly developed health care system, practically providing access to the drug to all patients who need.
Real-life data of Austrian RA patients treated with biologicals, Biosimilars, and Targeted Synthetic DMARDs (tsDMARDs) including JAKis have been collected in the BioReg Project database. BioReg, the Austrian registry for patients with chronic rheumatic diseases treated with these agents, was established in 2010. Meanwhile, more than 3100 patients from 28 centers have been enrolled into this database. The RA data core set encompasses joint counts, the Disease Activity score including an 28-joint count (DAS28-ESR), the Clinical Disease Activity Index (CDAI), and the Health Assessment Questionnaire (HAQ-DI), additionally, the Rheumatoid Arthritis Disease Activity Index-5 (RADAI-5) [17-21]. Moreover, CRP, ESR, RF, and anti-CCP are recorded, as well as comorbidities and adverse events. Female or male patients, treated with Biologicals, Biosimilars or tsDMARDs, older than 18 years, personally independent with inflammatory rheumatic diseases, such as RA, Psoriatic Arthritis, Ankylosing Spondylitis, and other diseases treated with Biologicals, Biosimilars and tsDMARDs, according to the licensed indications or generally accepted "off label” use can be included in BioReg. Personal dependence and withdrawal of informed consent are the only exclusion criteria. The ethics committee of Lower Austria has approved the study design of BioReg (reference number GS4-EK-085-2009) in 2009, and this approval is renewed annually [6]. All patients gave their written informed consent before inclusion into the registry. Data collection in BioReg started in 2010. For this study with a deadline of May 31st, 2019, the data of all RA patients receiving Bari or Tofa in the BioReg database were retrieved. Patient characteristics, including the most prevalent comorbidities, are given in detail in Tables 1 and 2. According to the primary goal of the registry, all safety issues and adverse events are documented, collected, and evaluated, not limited to treatment withdrawal. These are listed in Tables 3 and 4.
| Personal data | Baricitinib (n=74) | Tofacitinib (n=48) |
|---|---|---|
| Age (years; min-max) | 64 (26-84) | 64 (35-89) |
| Female sex | 80.30% | 87.50% |
| RF pos | 60.50% | 60.40% |
| ACPA pos. | 52,8% | 57,6% |
| Initial DAS28-ESR (mean ± SD) | 3.83 ( ± 1.2) | 3.80 ( ± 1.4) |
| Height (cm, min-max) | 166 (150-182) | 167 (153-183) |
| Weight (kg, min-max) | 74,7 (45-130) | 71.4 (43-119) |
| Duration (years; mean ± SD) | 14.1 ( ± 9.7) | 12.9 ( ± 9.39) |
| Biologic naive (n, %) | 14 (19%) | 8 (17%) |
| ≤ 2 biologics (n, %) | 32 (43%) | 34 (71%) |
| >2 biologics (n, %) | 28 (38%) | 6 (12%) |
| Co-morbidities (n, %) | 48 (65%) | 34 (71%) |
Table 1: Demographic data of the JAKI treated patients in BioReg.
| Co-morbidities Baricitinib (n=48, multiple answers possible) | |
|---|---|
| No co-morbidity recorded | 26 |
| Vascular diseases | 15 |
| Endocrine diseases | 14 |
| Heart diseases | 12 |
| Bone diseases | 8 |
| Psychiatric diseases | 6 |
| Pulmonary diseases | 6 |
| Gastrointestinal diseases | 1 |
| Co-morbidities Tofacitinib (n=34, multiple answers possible) | |
| No co-morbidity recorded | 14 |
| Heart diseases | 12 |
| Endocrine diseases | 12 |
| Vascular diseases | 11 |
| Bone diseases | 5 |
| Psychiatric diseases | 4 |
| Pulmonary diseases | 1 |
| Gastrointestinal diseases | 1 |
Table 2: Patients’ comorbidities.
| BioReg patients | Baricitinib (n=74) | Tofacitinib (n=48) |
|---|---|---|
| Duration of therapy (median, min-max) | 1.25 yrs. (0.25-2.5) | 1.25 yrs. (0.25-2.5) |
| Discontinuation of therapy (n, %) | 13 (17.6%) | 14 (29.2%) |
| Insufficient response* (n, %) | 24 (32.4%) | 21 (43.8%) |
| Adverse events* (n, %) | 16 (21.6%) | 12 (25.0%) |
Table 3: Therapy characteristics of patients in BioReg.
AEs Baricitinib (n=16) |
|
|---|---|
| Infections and parasitic diseases | 6 |
| Hematological diseases | 2 |
| Ear and labyrinthic diseases | 2 |
| Endocrine diseases | 1 |
| Genitourinary and breast diseases | 1 |
| Gastrointestinal diseases | 1 |
| Neurological disorders | 1 |
| Vascular diseases | 1 |
| Heart diseases | 1 |
AEs tofacitinib (n=12) |
|
| Infections and parasitic diseases | 5 |
| Gastrointestinal diseases | 2 |
| Ear and labyrinthic diseases | 1 |
| Skin diseases | 1 |
| Neurological disorders | 1 |
| Vascular diseases | 1 |
| Neoplasms | 1 |
Table 4: Adverse events in JAKi treated patients (MedRA-SOC terms).
In Austria, LAKIs are licensed for RA patients with moderate disease activity as expressed by an accepted disease activity scale (example, DAS28>3.2) after failure of at least one biological. Bari and Tofa are reimbursed in Austria since October 2017 and August 2018, respectively, in case of failure to one bDMARD. For this study, the following scores were retrieved from the registry: Disease Activity score including a 28-joint count (DAS28-ESR), Clinical Disease Activity Index (CDAI), and Health Assessment Questionnaire (HAQ-DI). Additionally, the Rheumatoid Arthritis Disease Activity Index-5 (RADAI-5) was documented [12-16]; not all instruments are applied in each patient, as the instruments used depend on the participant’s routine practice. Safety data were gathered according to MedDRA definitions and are depicted in a descriptive manner.
The registry comprised 1,408 patients with rheumatoid arthritis at the time of data retrieval, of which 74 patients were treated with Baricitinib and 48 patients with Tofacitinib together amounting to 8.7% of all RA-patients included in the registry. For analytic purposes, patient trajectories (“Spaghetti-blots”), depicting the course of the disease in an individual patient and mean value comparisons on a group level (as a conservative approach) were performed [22]. Propensity score matching with clinical trial data from the RA-BEACON study with Bari and the ORAL Step trial with Tofa [23-25] would have been a more stringent analysis, however, as we had no access to the raw data of the clinical trials, it was not possible to perform such an analysis. Thus, the results of the analysis of data obtained from BioReg were described in comparision data obtained from clinical trials with JAKis. Due to the inherent differences of data stemming from controlled trials versus registry data in terms of, among others, patient characteristics, timepoints, and frequency of control visits as well as the stringency of data completion, a descriptive rather than a statistical comparison was carried out and should serve to generate a hypothesis.
Seventy-four patients were treated with Bari, their mean age was 64 (26-84) years, 80.3% were female, and 60.5% were positive for Rheumatoid Factor (RF) and 52.8% for Antibodies against Citrullinated Protein (ACPA). Forty-eight patients were treated with Tofa, their mean age was 64 (range: 35-89) years, 87.5% were female, and 60.4% were RF and 57.6 were ACPA positive. JAKi treatment was initiated preferentially after one or two unsuccessful treatment attempts with biologics, namely, in 32 (43%) Bari treated patients, and in 34 (71%) patients treated with Tofa respectively, which is in line with the Austrian reimbursement regulations for these drugs. Fourteen pts (19%) received Baricitinib as their first treatment after csDMARDS failure, and 8 pts (17%) Tofa, respectively, see in Table 1. Comorbidities were evident in 48 pts on Bari (65%) and in 34 pts on Tofa (73%). The most prevalent comorbidities were vascular, endocrine, and heart diseases in Table 2.
The median treatment duration was 1.25 (0.25-2.5) years for both medications, [R] also the withdrawal rates (Bari 17.6%, Tofa 29.2%) were similar. After one year of treatment, approximately 80% of the patients were found still on JAKi therapy in Table 3. Safety data of the BIOREG registry were recorded according to the MedDRA classification system and are depicted in Table 4. Most frequent were infectious/parasitic diseases with a cumulative incidence of 8.1% for Bari and 10.4% for Tofa. According to the SOC terms, vascular disease occurred in one patient each on Bari or Tofa, and neoplasms were noted in one patient on Tofa Table 4. An insufficient response as assessed by the physician’s personal global evaluation was observed in 24 (32.4%) and 21 (43.8%) patients and adverse events occurred in 16 (21.6%) and 12 (25.0%) patients receiving Bari and Tofa, respectively Table 3 and Table 4. During the observation period up until the writing of this manuscript, none of the patients died or developed deep venous thrombosis. The DAS28-ESR was available for 50 pts. on Bari and 42 on Tofa, CDAI for 42/33, HAQ-DI for 57/40 pts., and RADAI-5 for 41/44 pts., respectively.
The mean baseline DAS28 was 3.83 ± 1.21 and 3.80 ± 1.41, respectively, for the Bari- and the Tofa-treated patients, with corresponding CDAI values of 15.7, and 20.3 ± 14.4. The respective RADAI-5 values were 4.4 ± 2.2 and 4.2 ± 2.4). All disease activity score values indicate moderate disease activity at the time of JAKi initiation, with mean HAQ values of 1.1 ± 0.7 and 1.2 ± 0.8 for the Bari- resp. Tofa-treated patients in Figures 1a and 1b. During the treatment period, all parameters showed improvement, which is demonstrated by the course of DAS28 for both drugs as, shown in Figures 2a and 2b. Mean analyses and patient trajectories, which outline the individual courses, showed the beneficial effects of JAKis on the disease course up to 24 weeks for Bari and 18 weeks for tofa. The analysis of the CDAI and RADAI-5 courses was similar, apart from some individual fluctuations as shown in Figure 3a and 3b.
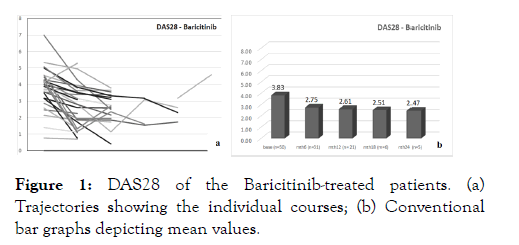
Figure 1: DAS28 of the Baricitinib-treated patients. (a) Trajectories showing the individual courses; (b) Conventional bar graphs depicting mean values.
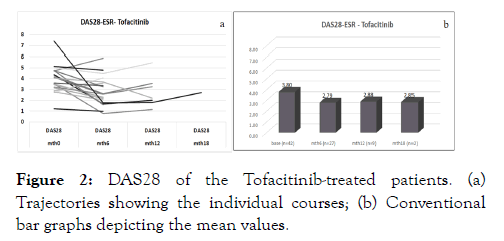
Figure 2: DAS28 of the Tofacitinib-treated patients. (a) Trajectories showing the individual courses; (b) Conventional bar graphs depicting the mean values.
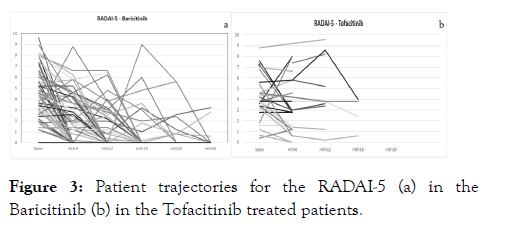
Figure 3: Patient trajectories for the RADAI-5 (a) in the Baricitinib (b) in the Tofacitinib treated patients.
Comparison with Bari in the RA-BEACON trial
The RA-BEACON trial was performed as a 6-month phase 3 clinical trial investigating the efficacy and safety of Bari in combination with csDMARDS in patients not adequately responding to prior therapy with a biological. This situation very much mirrors the characteristics of patients in BioReg treated with Bari, which was also the case in the Austrian patients included in the RA-BEACON study. [24]. In BioReg, 81% of the 74 patients treated with Bari had a treatment history of one or more biologics. Therefore, we compared the registry data with the patient group (n=177) treated with 4 mg Bari/day from the RA-BEACON trial [24]. Whereas sex distribution and disease duration were similar, patients in the BioReg were older (64 ± 12 vs. BioReg displayed less severe disease activity mean DAS28-ESR: 3.83 ± 1.2 vs. 6.6 ± 1.1, mean HAQ 1.1 ± 0.7) vs. 1.7 ± 1.6 compared to those in the RA-BEACON trial. The mean DAS28 and HAQ reductions at month six in the RABEACON trial were 1.7 and 0.4, respectively [24], whereas in BioReg, the respective reduction of DAS28 was 1.1, and the mean HAQ was found unchanged after six months. In the RABEACON trial, 88% of the patients completed the 6-month treatment, whereas in BioReg, 82.4% of the patients remained on Bari throughout the observation period, which lasted for 1.25 (0.25-2.5) years. The response rate, although difficult to compare in the clinical trial, was primarily assessed with the ACR criteria [26], whereas that in BioReg above all was assessed according to the physician’s discretion; 67.6% of the patients in BioReg were assessed to have a positive response, whereas in the RA-BEACON trial, only 30% achieved an ACR response of 50%. For the sake of better comparability, we also compared the percentage of patients achieving a DAS28ESR<3.2 at week 24. In BioReg, 48% (15 of 31) patients achieved the low disease activity threshold, while in the trial this percentage was 26%. Adverse events were more frequently reported in the clinical trial than in the registry (77% vs. 21.6%) and no new signals with respect to AEs were observed.
Comparison with Tofa in the ORAL-step trial
This study was performed as a 6-month phase 3 clinical trial investigating the efficacy and safety of Tofa in combination with MTX in patients with insufficient response to TNF inhibitors. This was also the case in 83% of patients BioReg treated with Tofa, suggesting similar patient populations in BioReg and the patient group (n=122) treated with 5 mg Tofa/twice a day in the ORAL step trial [25]. Gender distribution, RF positivity, and disease duration were similar in both groups, whereas the patients in BioReg were older (64 ± 12.2 vs. 55.1 ± 11.3) years and less active in their disease (DAS28-ESR 3.8 ± 1.4 vs. 6.5 ± 1.1, HAQ: 1.2 ± 0.8 vs. 1.6 ± 0.7) than those in the trial. The mean DAS28 and HAQ reductions at 6 months in ORAL step trial were 1.6 and 0.5, respectively [25], in contrast to 1.0 and 0.3 in BioReg. In the ORAL step trial, 80.5% of the patients completed the 6-month treatment, whereas, in BioReg, 70.8% of the patients remained on Tofa throughout the median observation period of 1.25 (0.25-2.5) years. Whereas 51.1% of the patients achieved an ACR response of 50% in the ORAL step one, 56.2% of the patients were evaluated by their treating physicians to have a sufficient response in BioReg. A DAS28ESR<3.2 at week 24 was achieved by 44% (12 of 27) in BioReg, in contrast to 25% in the trial. Adverse events were more frequently reported in the clinical trial than in the registry (42.9% vs. 25.0%). It is important to note that no new or unexpected adverse events occurred in the patients in BioReg.
The JAKis Bari and Tofa rapidly and very successfully found their position in the therapeutic armamentarium for RA [8]. This is also apparent in Austria with 8.7% of RA patients enrolled in BioReg two and a half years after licensing. In terms of gender and disease duration, patients in BioReg correspond to the patient population of the pivotal RA-BEACON and ORAL STEP ONE trials, however, BioReg patients are older, suffer from a higher number of comorbidities, and display a lower disease activity than the clinical trial cohorts. Altogether, data from the BioReg registry paint a reassuring picture of safety and affectivity similar/better to the ones in the pivotal trials. To translate data from successful clinical trials acquired from a highly selected patient population into clinical practice, data from registries, which include patients as comprehensively as possible, are important [27,28]. Due to the relative recent approval of Baricitinib and Tofacitinib by the European Medicines Agency in 2017, registry data pertaining to JAKi from Europe are limited and have mostly been presented at meetings [29] However, due to earlier approval in other states, sporadic real-life data have been published, e.g. from the US, Australia or Switzerland [22] The main scope of these reports was a comparison with other bDMARDs, in which the non-inferiority of JAKi compared to anti TNF known from clinical trials was underscored [12-14]. Mueller et al., on the other hand, investigated the safety of Tofa assessed by the reason to stop the medication and laboratory abnormalities. Again, clinical trial data were corroborated, and no new relevant safety signals were described [13]. The present report from the BioReg registry differs from most publications due to the comprehensive description of patient characteristics, safety data, and effectiveness data. Furthermore, the direct reference between clinical trial data and BioReg data provides additional insight beyond the data provided from other registries. Here we show that the efficacy of JAKi not only applies to the clinical trial population of patients in their mid 50ies with high disease activity but is used safely and effectively in patients>60 years old with only moderate disease activity reflected in the lower DAS28 values in BioReg vs. trials with Bari and Tofa [24,25]. In Austria, in contrast to many other countries, biological and tsDMARDs are usually initiated at this (rather low) disease activity stage [6]. The role of high DAS28 as a predictor of clinical response has been acknowledged in several trials of biological therapy [29, 30]. However, also in registry data with lower initial disease activity values, a significant improvement could generally be observed [31] a positive contribution of a lower baseline DAS28 to the achievement of low activity has also been observed in a Japanese report presented at the EULAR 2020 [32].
Altogether, patients in the trials as well as in the registry finally developed a low moderate disease activity on average, which was demonstrated in several disease scores. The BioReg data, also included the RADAI-5, not applied in the trials, which also mirrored the course of the composite indexes [15]. Additionally, visualizing the individual disease activity score courses by patient trajectories using BioReg revealed a beneficial course in most of the patients, despite some fluctuations. Patient trajectories may allow better insight into the course of the disease and may offer the opportunity to better estimate the individual patient’s chance for success [17]. The improvement of the HAQ values was less prominent in the registry than in the clinical trials, with lower baseline HAQ values, comparable disease duration despite higher mean age in the BioReg patients. Apart from the lower disease activity, which may have contributed to the lower HAQ score, the sociocultural variety in the participants of multinational trials may have played a role. At the end of the day, the JAKis proved to be effective to a remarkably similar extent, although without formal statistical comparison, in clinical trial population and the real world setting of the Austrian registry.
As expected, adverse events were more frequently reported in the clinical trial than in the registry (77% vs. 21.6%). Bari and Tofa were found to be well tolerated by the patients in the registry with an overall incidence of infection of approximately 10% as the most frequent adverse event. To our knowledge, this is the first report of any infection derived from registry data, and it implicates no new safety signals in the use of JAKi in real life. However, the strikingly higher rate of adverse events in the trials vs. the registry is evident and can-at least partly-be explained by the rigorous monitoring procedures of clinical trials, which cannot be guaranteed in registries.
There are some shortcomings of this study. The differences between clinical trials and BioReg are evident: In the registry, no close monitoring is performed; data are collected with differing frequencies and extent. However, the registries reflect the reallife setting, considering the above-mentioned shortcomings. The number of included patients in our Austrian registry is smaller compared to globally performed clinical trials or some other registries, but the evidence published in that respect is also founded on relatively small patient numbers [13,15]. However, the advantage of our registry data is the homogenous patient population with little sociocultural differences, a highly developed social insurance system, the unusual older age, and low disease activity of the BioReg patients. A caveat is the inclusion of all patients treated with JAKis, and not only biological non-responders [33]. However, we believe that following the new recommendations [34], JAKis will increasingly be used in biological naïve patients, justifying the inclusion of these patients.
Large, non-interventional observations, such as registries, seek to include as many patients as possible irrespective of comorbidities, age, disease activity, or other confounding factors, in contrast to the highly selected patient population of clinical investigations, to reflect daily practice as precisely as possible. Therefore, it is of particular importance for clinical practice to undertake efforts to verify or falsify the results of clinical trials by real-world evidence, which has been just recently strengthened by the German Institute for Quality and Economy in Health Care (IQWiG) [35]. Of course, propensity matching had constituted a method to achieve more robust results. However, the nature of the registry data and the impossibility to access the clinical trials’ raw data made it seem appropriate not to perform other than our primarily descriptive analysis. Registry data may serve as a possibility to generate hypotheses for future research. Such studies should comprise as many real-world data as possible as well as raw data from clinical trials with a propensity score analysis to validate our observations so that the efficacy and safety of JAKis is like clinical trial results and realworld data in RA.
In conclusion, for the Austrian patients enrolled in BioReg, the application of JAKis in clinical practice leads to results comparable to those of clinical trials, even though the patient population was older and had lower disease activity. This may contribute to an increased confidence of the treating physicians in the therapeutic properties of these drugs.
The ethics committee of Lower Austria has approved the study design of BioReg (reference number GS4-EK-085-2009) in 2009, and this approval is renewed annually (most recently 02/14/2020).
BFL Clinical trials: TRB, Roche, Consultancies: AbbVie, Amgen, Roche, MSD, Pfizer, Celgene, Grünenthal, Kwizda, Eli- Lilly, Novartis, Sandoz.
Speakers ‘bureau: AbbVie, Roche, MSD, Pfizer, Actiopharm, Boehringer-Ingelheim, Kwizda, Celgene, Sandoz, Grünenthal, Eli-Lilly.
RP: Consultancies: AbbVie, Amgen, Pfizer, Celgene, Grünenthal, Eli-Lilly; Speakers ‘bureau: AbbVie, BMS, Janssen, Kwizda, MSD, Pfizer, Celgene, Grünenthal, Eli-Lilly, PS, GES, MS, MH, FStS, RFS declared no conflicts of interest.
BioReg is a non-profit organization supported by ABBVIE GmbH; AMGEN GmbH, CELGENE GmbH; BIOGEN GmbH, ELI-LILLY GmbH, FRESENIUS-KABI Austria GmbH; JANSSEN-CILAG Austria GmbH, MERCK SHARP and DOHME GmbH; PFIZER CORPORATION AUSTRIA GmbH, ROCHE AUSTRIA GmbH; SANDOZ Pharma GmbH, UCB Pharma GmbH through an unrestricted educational grant. This study was supported by ELI-LILLY GmbH, Austria.
BFL: Contributed to data acquisition, design of the study, data analysis, manuscript writing.
PS, GES, MH, MS, RP: Data acquisition, data analysis, study design.
FSS, RFS: Data analysis, study design, manuscript writing.
Citation: Leeb BF, Spellitz F, Sturmc GE, Herold M, Stetter M, Puchner R, et al. (2021) Januskinase Inhibitors to Treat Rheumatoid Arthritis: Real World Data Match Clinical Trial Results an Evaluation by BioReg, the Austrian Registry for Biologicals, Biosimilars, and Targeted Synthetic DMARDS in the Treatment of Inflammatory Rheumatic Diseases. Rheumatology (Sunnyvale). 11: 288.
Received: 02-Jun-2021 Accepted: 16-Jun-2021 Published: 23-Jun-2021 , DOI: 10.35248/2161-1149.21.11.288
Copyright: © 2021 Leeb BF, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.