Journal of Horticulture
Open Access
ISSN: 2376-0354
ISSN: 2376-0354
Rapid Communication - (2019)Volume 6, Issue 2
Copper ions at proper levels are essential for plant cell growth and excess copper ions are toxic to root cell growth. The one meter height Theobroma cacao L. plants have roots in 10-15 cm depth nursery pots. “SpinOut®” high in Cu+2 and painted inside the plastic container mitigates against root encircling. Evidence is presented that a similar physicochemical effect can be obtained by incorporating copper ions into a keratin biopolymer matrix extruded into pellets and injection molded into a pot. Sustained controlled release of Cu+2 is obtained for at least six months. The same technology enables highly uniform delivery of the same amount of micronutrients self-consistently pot to pot. This approach would be useful in urban agriculture in which the biomass formulated composition of a soil matrix is especially non-uniform and in which both the plant and the surrounding soil matrix can be competing from among the same micronutrients.
Keratin nursery pots; Controlled release; Theobroma cacao; Cupric ions; Root bound plant; Environmentally friendly bioplastic
The National Chicken Council estimates U.S. broiler production in 2020 will nearly 20 x 109 kg in; in 2014 broiler production was 17 x 109 kg [1]. Since about 7% of the weight of each 2.2 kg chicken is feathers, a co-product of poultry production is nearly 1.4 billion kg of feather keratin. This abundant and sustainable agricultural biopolymer resource when shredded, formulated, and extruded, feathers form plastic films [2,3]. Feathers are an unusual biopolymer because they absorb both water and oil and will even absorb oil when wet [4].
Petroleum based polymers use about 0.7 billion kg of petroleum plastic for containers, primarily polyethylene (PE) and polypropylene (PP) [5]. For recycling, PE and PP are coded as different polymer types and should not therefore be co-mingled [6,7]. Uncertainty in the identifying any pot PE or PP is a serious complicating factor reusing the plastics regrinds to lower the costs of virgin PE or PP plastics. Fillers such as talc used in PE and PP agricultural plastics formulations limit the number of end product into which these mixtures can be recycled [8].
Cellulosic biocontainers have been considered a sustainable alternative to polymers made from synthetic polymers. However, low quality physical properties (e.g. strength, durability, and water-use efficiency (WUE) of these bioresins hindered end-product marketability [9]. Coating the surface of these pots with a biopolymer resin [10] has been used to improve WUE. Sufficient sustainable supply as well as raw material, engineering, and processing costs are also factors that affect end-product viability.
Composites of cellulosic materials and biopolymer resins can be formulated to optimize bioresin properties [11]. The positive surface and matrix effects of using feather keratin in place of cellulosic materials in composite formulations have also been examined to optimize end-product physical properties [12]. Carbohydrate fibers and commercial hydrocarbon-based resin containers release neither nitrogen nor any other plant nutrient. Because keratin is a naturally microbially degradable polyamide, surface keratin in pots can potentially be oxidized to nitrate or reduced to ammonia in soil. Multiple feather keratin composite formulation bioresins were injection molding to form nursery pots. Reports noting that specific keratin pots formulations containing feather biopolymers may affect the plant growth and development have been presented [13,14]. In this study, the formulated polymeric resin material by using feather fibers is designed to improve nutrition and root development of plants grown in containers. This suggests keratin bioresin in pellets and/or pots could be formulated to contain copper, calcium, iron, magnesium, manganese and/or zinc to provide sustained controlled release of such nutrients. Evidence is required to demonstrate mineral nutrients will progressively diffuse from specific pot formulations.
Copper (Cu+2) has been long proven to be an essential nutrient [15]. Biochemically, Cu+2 is essential in several plant enzyme systems including the chloroplast protein plastocyanin [16] and in the electron transport [17]. Horticultural studies found Cu+2 affects the growth of sunflower seedlings [18].
However, excess Cu+2 have been shown to inhibit growth in citrus rootstock [19] and in Theobroma cacao [20]. Excessive root growth is undesirable in potted nursery plants. Generally, container-grown plants develop circled and matted roots, a condition known as "root bound". Once transplanted, this can lead to poor root regrowth into the surrounding soil unless the root bound condition is corrected by mechanical root pruning prior to transplanting. Unfortunately, root- pruning causes transplant shock; where up to 80% of the root system can be damaged resulting in slower growth and sometimes death. Technologies for delivering levels of Cu+2 in nursery pots to control rootingout problem have been developed [21,22]. The commercial product ‘SpinOut’ (EPA Reg # 67690-26) contains 7.1% copper hydroxide and is painted on the inner surface of plastic nursery pots to inhibit root circling growth and promote root branching and compact root balls. The polymer matrix delivering Cu+2 ions requires both the proper dose and sustaining this dose over the relevant duration of time.
Synthetic polymers like PE and PP are hydrophobic and, though strong and durable, have no molecular binding sites with affinity for metal ions [23]. Thermoplastics and cellulosic materials can be formulated to contain metal ions dispersed in the polymer or biopolymer matrix [24]. However, limited and low specificity metal binding sites can result in minerals leaching out of containers with watering instead of being incorporated into the soil matrix. ‘Spin-Out’ avoids diffusion of metal ions from plants by having a two layer process in which the outside layer is water impermeable.
An alternative to a two layered pot is a single layer container in which the layer itself has affinity for metal ions. Copper ions have been shown to have affinity for wool keratin [25] and for protein peptide sites [26]. Keratin composites have been shown to incorporate and strongly retain metal ions at the molecular level [27]. Keratin in formulated composites has been shown to biodegrade microbially [28,29]. Thus both diffusion and slow biomatrix degradation are potential mechanisms of Cu+2 sustained release. In the present study, Cu+2 is formulated into a keratin composite resins which are then injection molded into nursery pot. Cu+2 levels in soil, leaves and stems are monitored after a six month time period with Spin-Out as a control. Dry weight of roots, leaves and stems is then used to evaluate degree of inhibition in the growth within plant fractions. Photosynthesis and chloroplast activity were also measured.
Previous research has reported plant nutrients and micronutrients incorporated into a keratin matrix can positively affect growth [30]. As the nursery pots biodegrade, nutrients bound to the pot matrix will be released as well. The design of a controlled release nursery pot cannot be optimized independent of the size and shape of nursery pot being produced.
Feather fiber biopolymer with copper (FFBP-Cu) nursery pot formulations
300cc capacity (7 cm diam. x 9 cm ht.) cylindrical FFBP-Cu nursery pots (Figure 1) were made containing 1%, 5% and 10% copper. For control and for ‘Spin Out’ treated samples, FFBP with no added copper nursery pots were utilized. Four circular holes in bottom of pots provide drainage.
Figure 1: Theobroma plants after six months Cu treatment.
The control FFBP with 0% Cu. ‘SpinOut’ (EPA Reg # 67690- 26; with 7.1% copper as copper hydroxide CAS# 20427-59-2; manufacturer: SePRO Corp., 11550 North Meridian Street, Suite 600, Carmel, IN 46032), was painted on the inside of an of control pots as directed.
Copper (Cu) (cuprous oxide) was purchased from Sigma Aldrich (Powder form; Product number: 61141) and was used in this form. The duck feather fibers were purchased from Downlite, Mason, OH 45040. The density value of this duck keratin fiber was 0.89 g/ cm3. In addition, ethylene acrylate copolymer (EA; e.g., Biomax® Strong) was used to improve the performance of the biodegradable materials. High density polyethylene (HDPE) was purchased from Adell Plastics, Baltimore, MD. Cuprous oxide under processing conditions converts to cupric oxide.
To produce the feather fiber resin pellets, for example, the composition was extruded at 90 rpm to 340 rpm with an extruder (NFM Welding Engineers, Counter Rotating Screw) with the extruder temperatures set at 226ºF to 241ºF (zone 1), 232ºF to 378ºF (zone 2), 238ºF to 395ºF (zone 3), 259ºF to 280ºF (zone 4), 272ºF to 320ºF (zone 5), and 300ºF to 340ºF (zone 6). The extruded material was granulated into pellets having lengths of about 11 mm. A pelletizer (C.W. Barbender Instruments, Inc., model BT25) was used to chop the strand material into pellets. Pellets are prepared from various formulations containing varying amounts of feather fibers, and other additives were needed to be mixed with the feather fibers (Table 1).
| Feather keratin pellets | Biomax Strong Additive | HDPE | Odor neutralizer | Metallic oxide (Cuprous oxide) |
|---|---|---|---|---|
| 30 | 9.4 | 59.3 | 0.3 | 1 |
| 30 | 9.4 | 55.3 | 0.3 | 5 |
| 30 | 9.4 | 50.3 | 0.3 | 10 |
Table 1: Feather fiber resin formulations (weight percentage).
After the extruded materials were granulated into pellets, the pelletized biodegradable materials were then molded using the injection molding technique (Niigata Model NE55-UA4; Year: 1996) in order to produce the feather fiber-Cu nursery pots. The nozzle temperature in the injection molder was set at about 220ºF to about 350ºF and the cylinder heating zones had to set point temperatures of about 195ºF to about 390ºF, and the mold temperature was set at 60ºF to 149ºF. The cooling time was about 5 seconds to about 60 seconds. The injection pressure was maintained at 1200 to 1800 psi.
Plant material and general culture
Pods collected from (Theobroma cacao L.) trees in a USDA/ARS-BARC greenhouse. The seed coat was peeled off, rinsed, soaked in 10% bleach for 5 min. and rinsed thrice in distilled H2O prior to planting. The growth media was sand:perlite1:ProMix1 (1Griffins GH Supplier LLC) (2:2:1 by volume) soilless mix.
The seeded pots were grown in a greenhouse that was maintained at a minimum of 18°C and a maximum of 29°C constant day/ night temperature, 50% automated shade cloth, and supplemental 400W HID lighting to provide a minimum of 50 W m-2 sec-1 and a 12-hour day length. The placement of pots on the greenhouse bench was randomized by generating a series of random numbers. The seedlings received regular watering and fertilizing regimens for greenhouse grown tropical woody species and grown for six months before harvesting.
Data collection and analysis
The experiment included five reps of five treatments with one seedling/pot per rep (N=25). Photosynthesis and chloroplast activity were measured with Li-Cor 6400 and SPAD meters, respectively. The plants were harvested into leaf, stem, and root fractions. Roots were rinsed to remove planting media; secondary roots were separated from the tap root. Weights of fractions plus leaf area were recorded. Plant samples were oven-dried for at least 48-hours at 60°C in a lab oven to obtain dried tissue weight. Dried leaf and stem samples that were larger than 2.0 grams was ground in a cyclone tissue grinder (make & model) and bagged in Zip-lok plastic bags for copper analysis.
Soil and plant analysis
Soil pH was measured in a 1:1 soil to water slurry using a combined electrode after 1 h; electrical conductivity was determined in 1:2 soil to water solution using a Orion conductivity meter after 1 h. The Mehlich-III method [31] was used to determine extractable copper; 2.5 g of each soil sample was placed in specimen cup and 25 mL Mehlich-3 solution added and shake horizontally at 200 oscillations per minute for 15 minutes. Solution was filtered using Whatman no. 42 filter paper. Copper concentrations in the solution were determined using a Perkin Elmer Inductively Coupled Plasma-Optical Emission Spectrometer (ICP-OES) with scandium as an internal standard. Copper composition of the leaf, stem and root tissue was determined by dry ashing in which two grams of plant sample was placed into 200 mL beaker and ashed at 480°C for 16 h. Two milliliter concentrated HNO3 was added to each beaker and allow to evaporation a hot plate. Ten milliliter of 3 N HCl was added to each beaker, cover with a wash glass and reflux for 2 h. The digest was filtered through Whatman # 40 filter paper and brought to 25 ml volume with 0.1N HCl. For quality assurance, one blank and one peach leaf standard from the National Institute of Standard Technology, Gaithersburg, MD was included for every 20 samples analyzed. Copper concentrations in digested solutions were determined using an Inductive Coupled Plasma Optical Emission Spectrophotometer (ICP-OES).
Cu+2 release from pots to soil
The Cu+2 content in keratin pot formulations from 0% to 5% after six months increased soil Cu+2 concentration from about 5% to 115% (Graph 1A). Despite the doubling of Cu+2 in pots to 10%, the soil level failed to even increase above that of 5% Cu+2 suggesting a maximum solubility of the ion in the potting soil matrix. At 1% and 5% of Cu+2, Cu+2 levels were retained in pot matrix. Additional studies can determine if different soil conditions and/or different soil matrices would alter the final mean Cu+2 level. Nevertheless, a 5% concentration of Cu+2 in the keratin pot matrix clearly was sufficient to maintain 115 ppm levels of Cu+2 in 300 cc volume nursery pots after six months. This maximum concentration at 5% Cu+2 suggests similar Cu+2 levels could be maintained even longer, e.g. a year, and that sufficient keratin bioresin capacity at 10% Cu+2 could be effective even in four liter nursery pots (Graph 1B and 1C).
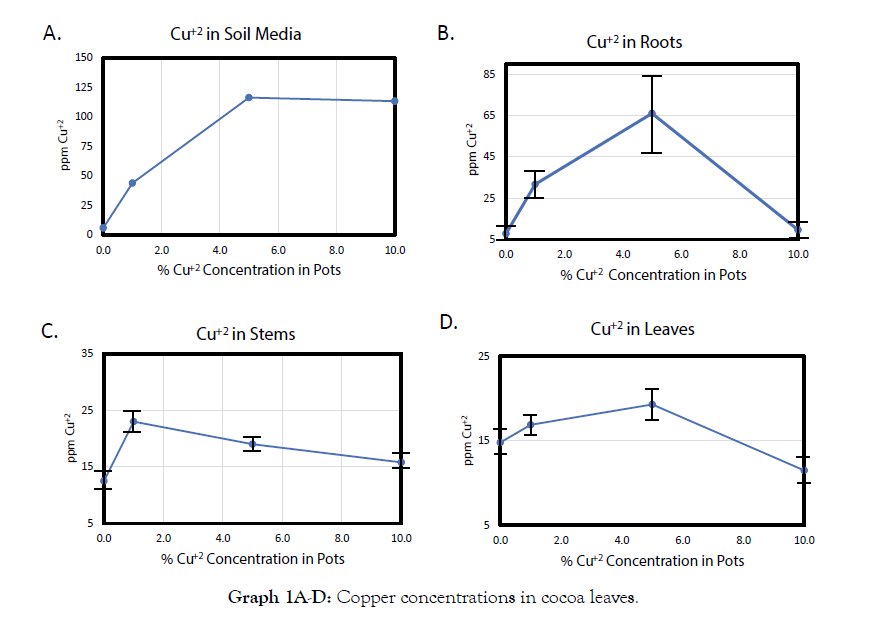
Graph 1A-D: Copper concentrations in cocoa leaves.
Copper concentrations in cocoa leaves (Graph 1D) were higher than the ranges (8-18 ppm) found in cocoa leaves [32]. Different soils composition/soil conditions in the field environment from which their samples were collected could explain this difference.
Even though cocoa leaves Cu concentrations were higher in this experiment, copper toxicity symptoms in whole plants were not observed (Figure 1).
However, since Cu+2 will be either an essential nutrient or a toxic heavy metal depending upon concentration, delivering Cu+2 in a sustainabled and controlable manner at a desired concentration can be a critical tool in meeting nutrient requirement in plants and avoiding toxic effects especially for plants grown in a controlled environment. The model of Cu+2 leaving the keratin bioresin matrix is a leaky faucet. Cu+2 will always leach from its higher concentration to sites with a lower Cu+2 concentration into the soil and the plant may or may not absorb available Cu+2 for physical chemical and/or biochemical reasons.
The levels of Cu+2 in roots (1B), stem (1C) and leaf (1D), are the concentration found in the plant fraction. In this case, root levels of Cu+2 increased significantly from 0% to 1% to 5% and strongly decreased to 10%. Stem Cu+2 levels were minimally affected by the treatments. Leaf Cu+2 increased similarly to root levels except to a lesser extent. Both the root and the leaf fraction appear to have a maximum Cu+2 uptake at 5% C treatment (115 ppm Cu+2 in soil). The higher level of Cu+2 in roots versus leaf suggest diffusion from higher concentration in roots to lower concentration in leaves is occurring. Because the mass of roots from 1% to 5% is only minimally increasing whereas the Cu+2 levels are increasing, the concentration of Cu+2 in root could be reaching a level that stunts root growth. In 10% Cu pots, the Cu+2 levels in Roots, Stems and Leaves were each similar (about 12 ppm) with no apparent Cu+2 gradient within the plant.
Diffusion from a higher concentration in the roots in 1% and 5% pots correlates with an increase in Cu+2 concentration in leaves. The increase in the Cu+2 concentration in 10% pots results in the lowering leaf mass. In contrast, a correlation for Cu+2 diffusion into stems from either roots or leaves was not evident and/or too weak to warrant significant conclusions.
The lower concentration of Cu+2 in sequence from pot to soil to roots to leaves supports that this process is primarily diffusion driven. The interfaces between adjacent sites however are major barriers to Cu+2 flow: the concentration Cu+2 inside the interface routinely fails to reach the levels outside the interface independent of the mechanism of action. Membrane fatty acid composition, for example, can be a biochemical mechanism which affects interfacial Cu+2 transport [33]. Evaluation of such interfaces and factors that affect specific interfaces is beyond the scope and intent of this research.
‘Spin-Out’ comparisons
Direct comparison between ‘Spin-Out’ and Cu+2 keratin pots were limited because the 7.2% Cu+2 results were not intermediate between 5% and 10% Cu+2 in keratin pots. The thickness of the coating and uniformity of the coating was not a variable controlled in this study. Moreover, since ‘Spin Out’ is designed to work at the surface of the pot, its mechanism of action can be very different from that of Cu keratin pots.
Cu+2 keratin pots durability
A steady state delivery of Cu+2 to plants from a pot matrix for six months requires the pots to be stable for over the same period. Water damage observed over time in cellulosic pots can be decrease by coating them with a polyurethane film [34]. After six months with plants growing in them, no water damage was observed in any of the keratin pots (Figure 2).
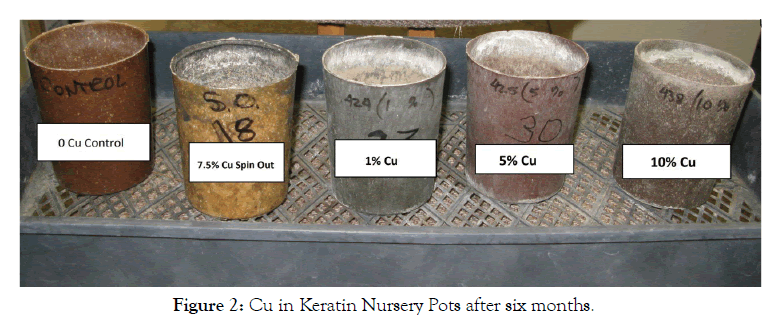
Figure 2: Cu in Keratin Nursery Pots after six months.
Interestingly, the color of each keratin pot changed with Cu+2 level. A small change in soil pH was observed with increasing Cu+2 concentrations. Adding Cu salts with different anions could alter Cu+2 release rates into the soil. However, a similar study would be required to determine if any such changes in release into the soil also resulted in changes in leaf Cu+2 levels.
The design of synthetic polymer nursey pots is to be uniform, strong, light weight, low cost and both physically and microbially inert. Potting soil composition is highly variable in physical properties such as porosity, surface area and macroscopic/microscopic uniformity in soil structure. Nutrient chemical composition is a variable because retention of nutrients in soils itself is a variable, and indeed, the soil matrix can be the largest source of variance in nutrient transport experiments.
In this unique experimental design, the nursery pot itself can be the source of a controlled release of nutrients or micronutrients. Analogous to a nicotine patch which allow a steady rate of nicotine release based into a patient’s blood stream, copper ions were incorporated into a nursery pot which allowed for a steady release rate of Cu+2 through the soil media to a Theobroma for a period of six months. The nursery pots require a composition that enables practical and workable rates of release over the period of the plant study. Since the amino acid composition of keratin is 50% hydrophilic, 50% hydrophobic, both hydrophilic and hydrophobic material can be incorporated into a keratin matrix such that the release rate proportional to its surface area and permeability. The uniformity of single nutrient composition among a lot of injection molded nursery pots will correlate with the precision of the uniformity nursery pot weight of less than 1%.
Horticulturalists routinely grow and group plants based on a uniform age and size; nursery pots can be formulated to release specific nutrients a well defined rate. The largest variable is interface between the two: the growth media/soil matrix in which the plants are to grow and the specific horticultural practices which can enhance the process. This experimental design enables rapid experimental access to collect data with nutrient release from within a specific production run of one formulation to be a constant not only over days but over months. Further, an assumption whether any specific soil is especially good or bad (or inert) for any individual plant can be quickly tested beginning from the same nutrient release rate and followed through the soil, roots, stems and leaves. This could be especially useful in home-gardening and urban agriculture situations in which specific newly added soil components may or may not be inert for any individual plant of interest.
The composition of any individual keratin nursery pot could be custom made to any horticulturalist specification except it would be rather useless without the arrays of scientific data of which concentration ranges would work best for which specific plants. Trace nutrients however may be incorporated into nursery pots for the same reason iodine is added commercially to salt: impractical to have each and every end user of salt to add iodine to their diet individually.
A little over a half of the mass of these nursery pots was composed of synthetic polymer resins to keep the polymer flow properties uniform during extrusion and injection molding. The formulated hybrid composition maintains its intended end product tensile strength and durability characteristics. The keratin fraction remains fully (microbially) biodegradable. Recently, a new class of GRAS (generally recognized as safe) plasticizers [35] was found which can enable the design of fully biodegradable bioresin formulations which can be processible in routinely used extrusion and injection molding polymer processing equipment without any synthetic polymer inputs.
Controlled release technology from a polymer matrix is established science [36,37]. This research suggests the source of the reservoir for micronutrients can instead be the nursery pot in which each individual plant is grown. Extrusion and injection molding can be formulated with an excellent level of uniformity. Optimizing nursery pot formulations to result in a constant rate of nutrient release over a period of at least six months. Further studies would be required to optimize nutrient release and release rates tailored to any other specific horticultural plant of interest.
A major intent of this research was to demonstrate nursery pots themselves could be an accurate and precise delivery device for delivering specific nutrients such as Cu to a plant. A full research article would have required a significantly larger number of plants and preferably more than one plant species to be able to warrant additional specific conclusions about Cu fate in any specific case. Data however does clearly show what one would expect from measuring a diffusion process: highest levels in pot diffuse to soil; higher levels in soil diffuse to roots; higher levels in roots to lower levels in stems; levels in stems and leaves similar.
Even in our data, nutrients passing through the soil into the Theobroma roots was clearly more variable than the levels in roots or in the pots. Technically, if one adds a variable initial amount of Cu to the soil, it can be very difficult to draw any statistically significant conclusions at all in the other data collected about Cu fate. Since soil is structurally complicated, both incorporation and release of nutrients from this matrix is correspondingly variable. Incorporating nutrients in the pot into which a plant is grow offer the possibility of precise control of the amount of nutrient added and an accurate controlled release rate of the nutrient.
The kinetics of Cu absorption in soil can thus be distinguished experimentally from the kinetics of Cu absorption in the plant. This technique can measure whether different soil types/matrices absorb or retain different levels of any specific nutrient prior to be being available to a plant and identify the time required in the same pot for this to occur.
Urban agriculture has been proposed as a pathway to increase worldwide food production [38,39]. The nutrient requirements of individual plants however are local and individual, not global. Presently the source of micronutrients in horticultural settings is from adding them via a water and/or soil matrix. Excess micronutrients added during watering adversely affect water quality downstream during runoff. Incorporating micronutrients uniformly into a soil matrix is a labor and/or machine intensive process. These techniques are not translatable to urban agriculture because the local soil matrix composition can be so highly variable and not be sustainably cost effectively uniform. Biodegradable pots can be used in green houses or “planted” as is outdoors in soil providing localized nutrients as the plant grows which minimizes or even obviates the need for fertilizing soil outside the plant pot.
Polymer Scientist Masud S. Huda developed, formulated, and optimized the hybrid polymer resins and injection molded the pellets into the nursery pots used in this study. Horticulturalist Martha E. Schmidt designed and ran the Theobroma horticultural protocol and for this six-month study. Research Agronomist Eton E. Codling conducted the mineralization of the plant fractions and performed the Cu analysis. Research Chemist Walter F. Schmidt was the Lead Scientist on this project.
Individual formulations of Nursery Pots from Feathers were developed under a Cooperative Research and Development Agreement (CRADA) between Agricultural Research Service (ARS) and Horticultural Research Institute (HRI) and evaluated under a Material Transfer Agreement with KeraBioTech.
None to report.
Mention of a brand name or trademark does not constitute endorsement of the product by USDA and does not imply its approval to the exclusion of other products that may also be suitable.
Citation: Codling EE, Schmidt ME, Schmidt WF, Huda MS (2019) Keratin Nursery Pots as Potential Medium for Controlled Release of Copper Ions in Root Growth Control in Theobroma cacao L. J Hortic 6:256. doi: 10.35248/2376-0354.19.06.256
Received: 24-Apr-2019 Accepted: 14-May-2019 Published: 21-May-2019
Copyright: © 2019 Codling EE, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.