Journal of Nutrition & Food Sciences
Open Access
ISSN: 2155-9600
ISSN: 2155-9600
Research Article - (2015) Volume 5, Issue 2
Enzymatic browning is engendered by conversion of monophenols (cresolase activity) and ortho-diphenols (catecholase activity) to reactive ortho-quinones in the presence of molecular oxygen by polyphenol oxidases (PPOs). The present study ascertained the inhibition kinetics of PPO extracted from Dioscorea rotundata tuber in the presence of non-toxic sulfhydryl amino acid (L-cysteine) and appraised other connecting kinetic and thermodynamic properties of D. rotundata PPO (DR-PPO). DR-PPO was extracted and purified by (NH4)2SO4 precipitation, ultrafiltration and dialysis. Thermodynamic parameters and DR-PPO activity were measured by standard methods. Kinetic analyses showed that 4.0 mM L-cysteine displayed non-competitive inhibition prototype with DR-PPO experimental substrate. The affinity of L-cysteine for DR-PPO inhibitor binding site was ≈ 25 folds greater than that of catechol for the enzyme active site. Arrhenius plot gave an approximate first-order reaction with activation energy (Ea)=60.07 kJ mole-1. DR-PPO activity upon incubation at T=50°C for 5 min resulted to 28.11 ± 0.05% decay in relative enzyme activity. Likewise, within experimental pH values (pH 7.5-10.5 units), decay in relative DR-PPO activity ranged between 19.39 ± 0.01% - 65.11 ± 0.05%. Furthermore, pH/DR-PPO activity profile displayed three peak values, which suggests the presence of DR-PPO isoforms. The present study propose the use of L-cysteine as an alternative inhibitor for DR-PPO activity alongside with thermal inactivation (T>70°C for 15 min) and pH adjustment (pH<7.5 units for 15 min) to alleviative and serve as control measures against enzymatic browning reactions in D. rotundata tuber.
Keywords: Arrhenius plot; Dioscorea rotundata; Inhibition; L-cysteine; Polyphenol oxidase
There are over 200 varieties of yam (Dioscorea spp) crops of which 10 species are sources of staple food in tropical regions of the world [1-3]. According to Sartie et al. [3] West Africa sub-region accounts for about 48 million tons of yam production annually on 4 million hectares of arable land. White yam (Dioscorea rotundata Poir.) is a major source of carbohydrates, vitamins and dietary fibers, often eaten boiled, fried and roasted or as pounded yam paste ‘Amala’ by the Yorubas. Yam and its products also serve as component of many confectionaries [4,5].
Polyphenol oxidase (PPO; EC 1.10.3.1) is a tetramer and coppermetallo enzyme with two binding sites for phenolic substrates including distinctly oxygen and copper binding sites [6,7]. For instance, field bean (Dolichos lablab) PPO is a homo-tetramer type III copper protein with a molecular mass of ≈ 120 kDa [8,9]. Enzymatic browning of plant tissues is engendered by the conversion of monophenols (cresolase activity) and ortho-diphenols (catecholase activity) to reactive orthoquinones in the presence of molecular oxygen by PPOs [10,11]. Consequently, quinones undergo auto-polymerization [9] and further nucleophilic attack by contiguous free amino acids, polyphenols and polypeptides to produce dark-brown or black pigments (polyphenolic melanins) that are observed in senescent and post harvested fruits and vegetables [10,12,13]. The browning reactions arise when plant tissues are traumatized or chewed out, engendering vacuolar phenolic substrates interaction with PPO localized in plastids and cytosol [14]. Thus, the browning reaction is initiated consequent upon physiologic or accidental tissue damage and disruption. PPO activity has a large effect on the quality of several fruit and vegetable crops, which engenders alteration of colour, flavour, texture and nutritional value [15-17].
Post-harvest handling, processing and storage of fruit and vegetable crops are usually associated with enzymatic browning [13]. Accordingly, post-harvest deterioration of the original white colour of white yam tends to discourage optimum utilization and acceptability of yam tubers and their finished products in both local and international markets [18]. Although PPOs are largely responsible for browning reactions, peroxidases (POD) and lipoxygenases (LOX) [19-21] have been implicated in the challenges and imperfections encountered in industrial/domestic preparation and processing of plant materials into finished food products.
Research endeavours into the prevention and control of PPO activity in plant materials is imperative because of its relevance in the food industry and domestic purposes. For several decades many research scientist have demonstrated the efficacy of diverse chemicals and physical control measures to impede the browning reactions in biological matters. Notable inhibitors of PPO are the citric acid, azides, chlorite, cyanides, mimosine, sulphated polysaccharides, aromatic carboxylic acid and dithiocarbamates [11,12,15,22] Ascorbic acids and related antioxidants react with the immediate PPO products (quinones) to form reduced compounds that are unsuitable substrate for the polymerization reactions. According to Landi et al., [23] the addition 5 mmol L-1 of ascorbic acid to lettuce (Lactuca sativa var. capitata L.) directly inhibited PPO activity by about 90%. Unfortunately, the browning reactions quickly ensue consequent upon the irreversible oxidation of ascorbic acid to dehydroascorbic acid during the reduction process thereby limiting the usefulness of the acid as an anti-browning agent [24].
Other chemical agents such as kojic acid, captopril {(2S)-N-(3- mercapto-2-methylpropionyl)-l-proline}, and thiopronine inhibit the browning reactions by interacting with both the catalytic site of PPO and immediate products of PPO catalysis sequel to the polymerization phase [11]. In another mode of action, many thiourea derivatives have been reported to exhibit the capacity to stall the browning reactions by impeding oxygen utilization. Specifically, phenylthiourea interacts with Cu2+ moiety at the active site of PPOs, engenders low oxygen utilization and thereby inhibits the browning reactions [11,25]. Unfortunately, according to Lu et al., [22] most of these well-known PPO inhibitors have been established to be toxic or potentially harmful to biologic systems and therefore come with obvious limitations in their application in the food industries.
Therefore, the present study ascertained the inhibition kinetics of PPO extracted from D. rotundata tuber in the presence of non-toxic sulfhydryl amino acid (L-cysteine) and appraised other connecting kinetic and thermodynamic properties of D. rotundata PPO (DRPPO). These investigations were in efforts to understanding the physiochemical properties of the enzyme with a view to providing insights into possible control and mitigation measures against the browning reactions in D. rotundata tuber.
Collection and preparation of plant material
Six (6) matured white yam (D. rotundata) tubers were harvested from a private botanical garden in Umoziri-Inyishi, Imo State, Nigeria on the 12th of September, 2012. The tubers were transported to the laboratory within 24 h after harvest. The tubers were identified and authenticated by Dr. F.N. Mbagwu at the Herbarium, Department of Plant Science and Biotechnology, Imo State University, Owerri, Nigeria. Thereafter, samples were washed under continuous current of distilled water for 5 min and air dried at room temperature (T=25 ± 5°C). The mid-sections of the 6 tubers were chopped up, rinsed in distilled water and stored at -4°C until used for analyses.
Extraction and purification of PPO
Extraction and partial purification of PPO was according to the methods of Madani et al., [26] with minor modifications as previously reported by Chikezie et al. [27] Ten grams (10 g) each of the 6 sample was homogenized in external ice bath, using Ultra-Turrax T25 (Janke and Kunkel, Staufen, Germany) homogenizer set in 80 mL of 0.20 M K2PO4/KHPO4 (phosphate buffer); pH=7.5 units containing 10 mM ascorbic acid (Darmstadt, Germany) for 180 sec at intervals of 15 sec. The homogenate was quickly mixed with 200 mL of acetone (Sigma- Aldrich, St. Louis, USA) (-20°C) to eliminate phenolic compounds [13] and squeezed through two layers of clean cheese cloth into a beaker kept in ice. The crude extracts were centrifuged at 32000 g for 20 min at 4ºC (Beckman J2-HS, USA). Solid (NH4)2SO4 (Sigma-Aldrich, St. Louis, USA) was added to the supernatant to obtain 80% (NH4)2SO4 saturation and precipitated proteins were separated by centrifugation at 32000 g for 30 min at 4ºC. The precipitate was re–dissolved in 10 mL distilled water and ultra-filtered in a Millipore stirred cell with a 10- kDa membrane (Millipore 8050, Milan, Italy). The filtrate was dialyzed in cellulose dialysis tubes (Sigma Chemical Co. USA) at 4ºC against phosphate buffer (pH=7.5 units) for 24 h with 4 changes of the buffer during dialysis. The dialyzed samples constituted the partially purified PPO extracts for enzyme assay. Protein concentration was measured by the method of Bradford, [28] using bovine serum albumin (Sigma- Aldrich, St. Louis, USA) as standard at λmax=595 nm. One unit of PPO activity (U) was defined as the amount of enzyme that causes an increase in absorbance of 0.001 mL-1 min-1 under the condition of PPO assay [29]. The measure of PPO purification protocol is summarized in Table 1.
| Enzyme Fraction | EA (U) | TP (mg) | Specific EA (U/mg) | PF | % Yield |
|---|---|---|---|---|---|
| Crude homogenate | 0.638 ± 0.04 | 0.880 ± 0.04 | 0.725 ± 0.03 | 1.00 | 100 |
| Centrifuged at 32000 g | 0.513 ± 0.02 | 0.098 ± 0.02 | 5.235 ± 0.03 | 7.22 | 80.4 |
| 80% (NH4)2SO4 | 0.324 ± 0.02 | 0.043 ± 0.02 | 7.535 ± 0.02 | 10.39 | 50.8 |
| Ultra-filtration | 0.287 ± 0.01 | 0.032 ± 0.02 | 8.969 ± 0.02 | 12.37 | 45.0 |
| Dialysis | 0.284 ± 0.01 | 0.026 ± 0.02 | 10.923 ± 0.03 | 15.07 | 44.5 |
Table 1: Purification steps of PPO extracted from D. rotundata tuber.
Measurement of PPO activity
PPO activity was measured immediately after the extract was partially purified. Enzyme assay was according to the methods of Qudsieh et al., [30] with minor modifications according to Chikezie et al. [27] Enzyme activity was determined by a measure of increase in absorbance the analyte using a spectrophotometer (U-2000 Hitachi, Japan) at 25 ± 5ºC. The reaction mixture contained 3.5 mL of 0.20 M phosphate buffer (pH=7.5 units), 1.0 mL of each serial dilutions of 4-16 mM catechol, and 0.5 mL of enzyme solution in a final volume of 5.0 mL. The mixture was quickly transferred into a cuvette and the change in absorbance was monitored at a regular interval of 0.5 min at λmax=540 nm. The rate of the reaction was calculated from the initial linear slope of activity curves. PPO activity was measured as rate of change in absorbance (Δ OD min-1) of the enzyme assay mixture. Furthermore, by extrapolations, standard catechol plot was used to measure the concentrations of catechol (mM) consumed during the enzyme assay.
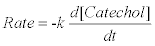
Where k=rate constant.
Measurement of temperature and pH optima of PPO activity
Activity of PPO was measured in assay mixture containing 16 mM catechol under varying temperatures within the range of T=10- 55°C; at pH=7.5 units. The enzyme activity was measured using 0.20 M phosphate buffer, adjusted with 0.1 M NaOH or 0.1 M HCl, [10] within the range of pH=6.5-11.5 units; at T=25°C.
Inhibition studies
The enzyme assay was carried out in the presence of 0.20 M phosphate buffer (pH=7.5 units)/L-cysteine (Sigma-Aldrich, St. Louis, USA) mixture (2:1; v/v). Final concentration of inhibitor=4.0 mM.
Evaluation of kinetic constants
The Km and Vmax values of PPO were measured by evaluation of the Lineweaver–Burk (1/Vo versus 1/[S] values) plots [31].
Thermal and pH stabilities of PPO
The effect of temperature and pH on PPO activity as a measure of enzyme stability was ascertained according to the methods of Chikezie et al. [27] Purified enzyme solutions extracted from D. rotundata tuber were stored in capillary tubes (1.0 mm i.d., 200 μL total volume) and held on ice until pre-incubated in a circulating water bath (Model 20B, Julabo, Allentown, PA) [19] at temperatures of 50, 60 and 70°C. At regular time intervals of 5, 10 and 15 min, aliquots of the enzyme solution was withdrawn and assayed for PPO activity. The residual PPO activity was measured according to the modified methods of Mizobutsi et al., [32] at pHoptimum ≈ 8.5 units and Toptimum ≈ 30°C, at the given time intervals. At the same time intervals, measurements of PPO activity of enzyme extracts pre-incubated at varying pH values of 7.5, .9.5 and 10.5 units were carried out. The residual PPO activity was measured accordingly at pHoptimum ≈ 8.5 and Toptimum ≈ 30°C. Residual PPO activity was reported as the relative PPO activity (%) to control PPO activity of extract solution at corresponding temperature and pH optima of assay conditions.
Statistical analysis
The experiments were designed in a completely randomized method and data collected were analyzed by the analysis of variance procedure while treatment means were separated by the least significance difference (LSD) incorporated in the statistical analysis system (SAS) package of 9.1 versions, (2006). The R2 values and equations of the kinetic plots were analyzed by Microsoft Office Excel, 2010 version.
Enzyme assay of the partially purified PPO extracted from D. rotundata tubers showed that the enzyme activity profile exhibited classical non-competitive inhibition kinetics in the presence of 4.0 mM L-cysteine (Figure 1). In addition, numerical values of DR-PPO kinetic constants (Km and Ki) indicated that the affinity of L-cysteine for the enzyme inhibitor binding site was ≈ 25 folds greater than that of catechol for the enzyme active site (Km>Ki).
Calculations
Evaluation of Km value;
-1/Km=intercept of the control plot on the x-axis (Figure 1) (2).
-1/Km=-0.073 mM-1
ð Km=13.70 mM
Evaluation of Vmax;
Given: y=0.5545x + 0.0404; control plot (Figure 1) (3).
Where y=1/Vo; x=1/[S]
But 0.0404 (Δ OD min-1)-1=intercept of the control plot on the y-axis
Therefore, 1/Vmax=0.0404 (Δ OD min-1)-1
ð Vmax=24.75 Δ OD min-1
Evaluation of Ki;
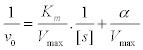 (4)
(4)
Where: 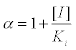
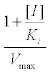 =intercept of the test plot on the y-axis (Figure 1).
=intercept of the test plot on the y-axis (Figure 1).
Given: y=0.958x + 0.0704; control plot (Figure 1) (5).
Thus: 0.0704 (Δ OD min-1)-1=intercept of the test plot on the y-axis
Thus: 0.0704 (Δ OD min-1)-1=intercept of the test plot on the y-axis
ð Ki=0.539 mM.
Physicochemical indicators of DR-PPO showed that the enzyme activity gave pH optimum ≈ 8.5 units and Toptimum ≈ 30°C. Both the pH and temperature dependent DR-PPO activity profiles exhibited the characteristic dumb-bell enzyme activity configuration (Figures 2 and 3). A cursory look at Figure 2 showed three peaks in the pH depended DR-PPO activity profile at pH ≈ 8.5 units, pH ≈ 10.5 units and pH ≈ 11.5 units. DR-PPO activity was not completely inactivated within the experimental pH values (pH=6.5-11.5 units). An overview of Figure 3 revealed rapid increase in DR-PPO activity between 15°C and 25°C, compared with the corresponding phase of slow declining DR-PPO activity when T>30°C (Toptimum ≈ 30°C); precisely within the ranges of 30°C ≤ T ≤ 40°C and 45°C ≤ T ≤ 55°C. Specifically, DRFigure PPO activity at T=25°C (VO=0.923 Δ OD min-1) was not significantly different (p>0.05) from enzyme activity at T=30°C (VO=0.934 Δ OD min-1). Likewise, the enzyme activity was not completely inactivated within the experimental temperature conditions: 10°C ≤ T ≤ 55°C. Standard errors (SEM) are shown as error bars on the data points in the Arrhenius plots.
From the Arrhenius plot (Figure 4), at T>≈ 27.5°C (i.e., at 1/ T>0.003327 K-1) the inactivation rates increased logarithmically with temperature reciprocals (K-1). Therefore, the plot can be approximated to a simple first-order reaction.
The activation energy (Ea) of catalysis was evaluated thus:
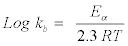 (6)
(6)
Where R is the gas constant 8.314 J mol-1 K-1; T is the temperature in Kelvin (K).
But gradient of the ascending plot is: 3141.5929 = 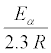
Therefore, Ea=60.07 kJ mole-1.
Decimal reduction time (D value), which is defined as the duration (t min) to reduce DR-PPO activity by 10% of its original value by thermal inactivation is given as:
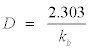 (7)
(7)
Evaluation of eqn 7 showed that D-value of DR-PPO gave 13.81 min at T=55°C whereas, at T=30°C, D-value was=19.78 min. At relatively lower temperature value (T=10°C) D-value was 3.97 min.
The half-life of PPO (t1/2) was calculated thus:
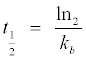 (8)
(8)
Where kb=inactivation constants.
From eqn 8, at T=30°C, the half-life (t1/2) of DR-PPO was t1/2=5.95 min and at T=55°C, t1/2=4.16 min. From the Arrhenius plot of inactivation rates (Figure 4), the Ea value of DR-PPO in the presence of catechol was calculated to be=60.07 kJ mole-1. An overview of Table 2 showed that the activity and stability of PPO extracted from D. rotundata decreased in proximate proportionality to the duration and temperature of DR-PPO incubation. For instance, DR-PPO activity upon incubation at T=50°C for 5 min resulted to 28.11 ± 0.05% decay in relative enzyme activity. The highest level of decay in DR-PPO activity occurred when the enzyme was incubated at T=70°C for 15 min, which represented 88.66 ± 0.03% decay in DR-PPO activity.
| Relative PPO activity (%) | |||
| Time (min) | 5 | 10 | 15 |
| T = 50 °C | 71.89 ± 0.05 | 59.45 ± 0.03 | 47.22 ± 0.04 |
| T = 60 °C | 54.66 ± 0.03 | 45.02 ± 0.04 | 40.62 ± 0.05 |
| T = 70 °C | 21.03 ± 0.04 | 18.39 ± 0.03 | 11.34 ± 0.03 |
Table 2: Residual D. rotundata PPO activity incubated at varying temperature.
Values are mean ± S.D of 6 determinations. PPO activity=0.934 ± 0.04 Δ OD min-1 at pH=8.5 units; T=30°C.
Residual DR-PPO activities under varying incubation pH conditions were in the order of 9.5>10.5>7.5 units (Table 2). Generally, within the experimental pH values (pH 7.5-10.5 units), decay in relative DR-PPO activity ranged between 19.39 ± 0.01% - 65.11 ± 0.05%.
By comparative inspections, DR-PPO was relatively more stable under varied pH conditions than the thermally adjusted incubation conditions, exemplified by the higher residual DR-PPO activity (Table 3). Specifically, changes in temperature from 50 to 70°C caused greater loss of DR-PPO stability than that observed when the pre-incubation pH level was increased from 7.5 to 10.5 units. In addition, preincubation of the enzyme extract at pH=9.5 units for 5 min elicited comparatively poor decay in DR-PPO activity (relative activity=19.39 ± 0.01%).
| Relative PPO activity (%) | |||
| Time (min) | 5 | 10 | 15 |
| pH=7.5 units | 41.61 ± 0.04 | 38.01 ± 0.03 | 34.89 ± 0.05 |
| pH=9.5 units | 80.61 ± 0.01 | 75.04 ± 0.03 | 73.89 ± 0.04 |
| pH=10.5 units | 61.44 ± 0.03 | 55.56 ± 0.03 | 51.67 ± 0.02 |
Table 3: Residual D. rotundata PPO activity incubated at varying pH.
The outcome of the extraction and purification protocol of DR-PPO (Table 1) measured up with those described elsewhere, [33,34] exemplified with the relatively satisfactory enzyme yield and purification fold of the final extract cocktail (Table 1). In practice, the relative enzyme yield and purity depends on the cultivar and origin of the plant material in conjunction with the techniques of extraction protocol. Generally, the kinetic properties of PPOs have been reported in previous studies of several plant tissues, [16,22,33-36] which, by and large, measured up with the present observed kinetic indices of DRPPO. Notwithstanding, the Km value of DR-PPO (Km=13.70 mM) in the presence of the experimental substrate (catechol) was comparatively higher than to those of Jerusalem artichoke (Helianthus tuberosus) PPO: Km=5.09 mM, [33] apple (Malus pumila) PPO, using 4-methyl catechol and pyrogallol substrates: Km=2.24 mM and 8.04 mM, respectively [16]. In contrast, DR-PPO Km value was comparatively less than mulberry (Morus alba L.) PPO: Km=19.81 mM [10]. The values of Vmax of DR-PPO were proportional to the experimental substrate and enzyme concentrations in the assay mixture. Overall, studies have shown that the experimentally derived kinetic constants were contingent upon purity of the enzyme assayed, which in turn depended on enzyme extraction/purification protocol, molecular properties of experimental substrate and presence of activators/inhibitors [36-39].
The mode of inhibition of L-cysteine visa-a-vise the tendency of the inhibitor to compete with the substrate for PPO binding sites conformed to the classical non-competitive inhibition pattern as previously reported elsewhere [13,17,40]. Furthermore, kinetic evaluation showed that DR-PPO Ki value, which is a measure of affinity for inhibitor binding sites, in the presence of L-cysteine was comparatively higher than that of mushroom (Agaricus bisporus; J.E. Lange) PPO treated with a competitive-type inhibitor; [benzoic acid]=0.05 mM; Ki value=0.046 mM [41]. The present study showed that L-cysteine binding capacity with DR-PPO was in equivalent dimension to cassava (Manihot esculenta Crantz) leaf PPO, using catechol as the experimental substrate [17]. Also, Ki value of the present study was ≈ 68 folds lower than that of ferulic acid, which exhibited non-competitive reversible inhibition kinetics towards PPO extracted from cephalothorax of Pacific white shrimp (Litopenaeus vannamei) [42]. Studies by Robert et al., [43] gave the inhibition constants of the following inhibitors of palmito (Acanthophoenix rubra) PPO to be: benzoic acid; Ki=0.14 mM, cinnamic acid; Ki=0.019 mM and sorbic acid Ki=0.15 mM. By comparative analyses, the experimental derived Ki=0.539 mM of PPO extracted from D. rotundata at [L-cysteine]=4.0 mM, was an indication that L-cysteine could serve as an effective inhibitor against enzymatic browning reactions in D. rotundata tuber as represented by previous studies of PPO inhibition kinetics of other plant genera [10,12,34,40,44-46]. In synergy with the direct inhibitory actions on PPO activity, L-cysteine by virtue of its sulphydryl group, which is a strong nucleophile, forms colourless addition o-quinones derivatives or/and facilitates the reduction of quinones back to their corresponding phenol substrates [7,10,47]. These reported inhibitory potentials notwithstanding, Gacche et al., [12] noted that L-cysteine is a time bound inhibitor of apple (Malus pumila) PPO in that inhibition of the enzyme activity by 5 mM L-cysteine was only effective within the duration of 4 h.
The relationships between Ea of plant enzymes and their corresponding thermo stability have been reported by several researchers, [19,20,36,40,48-50] in which they noted that relatively high enzyme Ea value was indicative of high thermo stability. Particularly, Fortea et al., [36] stated that PPO and POD extracted from table grape (Crimson Seedless) exhibited similar thermo stability, exemplified by their comparable Ea values; PPO=295.5 kJ mol-1 and POD=271.9 kJ mol-1. For the most part, the Ea values of PPOs from multitude of previous research reports showed wide disparities with that reported here and those elsewhere. These differences may arise as a result of fluctuations in levels of enzyme purity and certain intrinsic physicochemical properties such as thermo stability of the enzyme and composition of the surrounding solution of the enzyme during heat treatment [19,20,51].
The activity profile of DR-PPO under varied temperature and pH conditions conformed to the inverted bell-shaped curve as earlier described [13,17,27,52]. The pH versus DR-PPO activity profile displayed peak values of enzyme activities at pH ≈ 8.5 units, pH ≈ 10.5 units and pH ≈ 11.5 units (Figure 2), which were obvious indication of the presence of isoforms of DR-PPOs, based on previous propositions [21,27,38,52-57]. The study by Altunkaya and Gökmen, [21] showed the presence of several PPO isoforms that have been implicated in browning reactions. The LOX1 and LOX2 isoforms designates gave pH optima at 6.0 and 7.0 units, respectively. They further noted that PPO isoforms assigned as PPO1, PPO2, PPO3 and PPO4 eluted at pH values of 4.25, 4.5, 4.75 and 8.75 units, respectively. According to Onsa et al., [58] the pH optima of POD from lettuce were reported to be between pH=6.0 and 8.5 units.
One of the notable peak values or pH optima of DR-PPO activity (pH ≈ 8.5 units) as reported in the present study differ from amongst other several plant PPOs pH optima previously mentioned in literatures. For instance, pH optimum of litchi (Litchi chinensis Sonn.) pericarp PPO was ≈ 7.5, [13] H tuberosus skin PPO pH optimum ≈ 7.5 units, [40] strawberry (Fragaria spp) fruits PPO pH optimum ≈ 4.0- 4.5 units [49] and Thompson seedless grape (Vitis vinifera) PPO pH optimum ≈ 6.0 units [46]. Notwithstanding, the pH/activity profile of DR-PPO of the present study matched that of Mahmood et al. [55] The reports of Mahmood et al., [55] noted that apricot and apple PPOs exhibited substantive activity at neutral and alkaline pH, whereas the activity profoundly declined at pH<7.0 units as was the case of Koshu grapes PPO [59]. Studies have established the fact that variations in pH optimum of PPO activity depended largely on the plant maturity and cultivar, [40,49,55] in concert with experimental factors such as extraction methods and purities of enzyme, presence of activators/ inhibitors in addition to the experimental buffers and substrates used for analyses [9,49]. In general, most plants exhibit maximum PPO activity near neutral pH values [13,27,60].
Temperature optimum of DR-PPO activity (Toptimum ≈ 30°C) defines the maximum enzyme activity attainable under a range of varying experimental temperature conditions (T=10-55°C). From previous investigations, temperature optima of PPOs extracted from varieties of plants have been established to vary between values of 20- 50°C [17,44,47,61-63]. However, few evidence have shown that PPO extracted from the same plant tissue may exhibit diverse T°Coptimum
depending on the nature of the experimental substrate used for the enzyme assay [10,40,55] as well as physiologic characteristics such as the cultivar, stage of maturation and presence of isoenzyme [53]. The present study showed that Toptimum ≈ 30°C of DR-PPO activity corresponded with PPOs extracted from spearmint (Mentha arvensis), [63] banana (Musa sapientum L.) pulp, [44] and tea leaf (Camellia sinensis) [47]. Studies have also shown that enzymes are less thermostable at higher temperatures, which was consistent with the present findings, exemplified by the increasing first order inactivation rates and decreasing D-values of DR-PPO with increasing temperature conditions. Decay in DR-PPO activity amplified with increase in incubation time and temperature conditions (Table 2), typified by reduction in the calculated corresponding half-life of the enzyme (Equation 7). Thermal treatment (T>70°C for 15 min) of DR-PPO showed strong evidence of substantial denaturalization of the enzyme that was comparable to that of L. chinensis Sonn [13] and pawpaw (Asimina triloba) fruit [52]. Furthermore, previous reports of Yemenicioglu et al., [53] on Taro (Colocasia antiquorum) PPO corroborated the thermostability dynamics of DR-PPO of the present study. The enzyme structure/activity relationship suggests that the stability of DR-PPO was a reflection of its level activity, which was inextricably connected with the functional three dimensional structure of the enzyme. From general concepts, relatively high non-physiologic thermal energy level engenders increase in kinetic energy of enzyme molecules to values that exceed the activation energy barrier for disrupting noncovalent interactions (hydrogen bonds, van der Waals, hydrophobic and hydrophilic forces) that sustain their three-dimensional structures. Consequently, polypeptide chains of the enzyme unfold or are denatured with concomitant loss of catalytic activity. Typically, most enzymes maintain a stable catalytically active conformation at temperature values within or moderately above that of the cell in which they reside [64]. The thermo stability properties of PPOs extracted from several varieties of plants have been extensively discussed elsewhere [27,40,49,55,65].
Likewise, the influence of hydrogen ion (H+) concentration (pH) on stability and functional three dimensional structures of enzymes are well established [64]. Pre-incubation of PPO extracted from different segments of the same plant organ and different plant genera have revealed disparities in the capacity of PPOs to withstand chaotropic potentials of extreme pH conditions [27,32,54]. Contrary to the present study, Liu et al., [13] noted that L. chinensis Sonn pericarp PPO (pHoptimum=7.5), using (-) epicatechin as substrate, gave residual activities of 86.25, 86.31 and 80.17% after 67 days low temperature (T=4°C) incubation at pH values of 6.0, 7.5, and 8.0 units, respectively. The relatively high residual activities of L. chinensis Sonn pericarp PPO was the outcome of the low pre-incubation temperature and proximity of the pre-incubation pH conditions to pH optimum of the enzyme. However, their findings showed that at relatively low pH preincubation conditions, L. chinensis Sonn pericarp PPO followed the inactivation pattern of DR-PPO activity (pH<7.5 units). They further noted that pre-incubation of L. chinensis Sonn pericarp PPO at pH=3.1 units for 1 day caused 49.50% loss in enzyme activity, whereas only 2.43% of the activity remained after 12 days of incubation, indicating that L. chinensis Sonn pericarp PPO was very unstable at pH=3.1 units.
The present study propose the use of L-cysteine as an alternative inhibitor for DR-PPO activity alongside with thermal inactivation (T>70°C for 15 min) and pH adjustment (pH<7.5 units for 15 min) to alleviative and serve as control measures against enzymatic browning
The author is grateful for the technical assistance offered by Mr. O.A.K. Emenyonu, Chief Laboratory Technologist, Department of Biochemistry, Imo State University Owerri, and Nigeria.