Journal of Physical Chemistry & Biophysics
Open Access
ISSN: 2161-0398
ISSN: 2161-0398
Research Article - (2014) Volume 4, Issue 2
The kinetics of decolorization of methylene blue (MB+) with sodium sulphite in aqueous media was investigated over the temperature range 20-40°C. The kinetic studies were carried out as a function of different variables like concentration, pH, and temperature on rate of decolorization. The rate of the reaction was found to be [H+] dependent and first order in both [MB+] and reductant. The empirical rate law conforms to the equation:
Where K1 is the protonation constant, and k2 and k3 are overall pseudo-second order rate constants for the decolorization of MB+ with 2 3 SO − . The rate was found to increase linearly with temperature. The rate of decolorization decreased after addition of Co(II), Ni(II), Mn(II) and Zn(II), but increased after addition of Fe(II) and Cu(II). The activation parameters (Ea, ΔG#, ΔH# and ΔS#) of the decolorization reaction of MB+ with 2 3 SO − in absence and in presence of Fe(II) and Cu(II) were calculated.
<Keywords: Methylene blue; Sodium sulphite; Metal activation; Kinetics; Decolorization
A variety of hazardous pollutants are discharged into the aquatic bodies from several industrial streams [1,2]. Dye from textile industries and other commercial dyestuffs have been a focus of environmental remediation in the last few years [1,3,4]. The use of methylene blue as a dye in textile industry [5] has decreased slightly in last few years due to its high light sensitivity. However, it is still widely used for the manufacture of color pens and polygraphic inks [6]. The color and toxicity which dyes impart to water bodies are very undesirable and harmful to the water users for aesthetic and environmental reasons [7,8].
Methylene blue (MB) is a cationic dye, used extensively for dying cotton, wool and silk. The risk of the existence of this dye in waste water may be arisen from the burns effect of eye, nausea, vomiting and diarrhea [9,10]. Generally, several methods have been developed for the removal of dyes from effluents including precipitation, adsorption, and reverse osmosis, aerobic and anaerobic treatment, oxidation and reduction [11-19]. To find a general process for treatment of the color of dye used in dyeing processes is very difficult due to the complexity and variety of these types of industrial wastewater. The disadvantages of these methods are sludge formation, waste disposal and high operation cost, time consuming and ineffectiveness in cases where complicated aromatic compounds are present [20,21].
Literature is rich in articles about kinetic studies of the oxidation of MB+. Studies on redox reaction of methylene blue are mainly focused on the kinetics of transformation into leuco-MB [22,23], whereas the kinetics of its reductive decolorization in the presence of transition metal ions have been very scanty. The decolorization can be obtained by SCN– (partial decolorization) [24], H2O2 [25,26], UV/H2O2 [27,28], ascorbic acid [29-32], strong oxidant such Ce (IV) [33], KBrO3 [34,35], KClO3 [36], O3 [37], UV/O3 [38], activated O2 [39], Fenton’s reagent [40-42], photo Fenton [43], persulfate [44], photodecomposition on TiO2 [45,46], Br2 in basic medium [47], sulphide (Na2S) [48], and finally, reducing sugar [49]. Sulphite which sometime plays the role of oxidant and sometimes the role of a reductant, is employed in this study. The preliminary test showed the addition of sulphite (Na2SO3) in excess to a MB+ solution faded its blue color with time, and therefore interesting to deeply study the kinetics and parameters affecting this decolorization. Also investigated, is whether the addition of small amount of transition metal ions influence decolorization rate of methylene blue with sulphite. The red-ox properties of methylene blue make a useful indicator in analytical chemistry [50]. Methylene blue is blue when it is in an oxidizing environment, but colorless (leuco methylene blue (LMB)) if it is exposed to a reducing agent (Scheme 1).
These processes have found applications in numerous inventions like data recording holographic industries, optical data storage, food and pharmaceutical industries. Such reduction is also used in checking the purity of milk [51,52].
In the present study, an attempt is made to investigate the decolorization kinetics of methylene blue with sodium sulphite in aqueous media by UV-Visible spectrophotometry. The effect of various parameters such as initial pH, initial concentration of sulphite, MB+ and transition metal ions concentration was studied.
All chemical reagents used were of analytical grade. Methylene blue was obtained from M & B Laboratory Chemicals in the laboratory grade and used without further purification. Distilled water was used to prepare all solutions. A stock solution of Na2SO3 and MB+ were prepared by dissolving the required amount in distilled water. A Na2SO4 solution was added to reaction mixture in order to maintain the ionic strength of the solution. The effect of pH was also studied by adjustment of pH of the reaction mixture prior to decolorization with NaOH or HCl and measured by a pH meter (JENWAY 3505 pH meter). The effect of temperature was studied and the temperature was controlled by a horizontal thermostated shaker (SM 101 by Surgafriend Medicals). The absorbance of the reaction mixture at different reaction times was recorded by measuring the absorption intensity at 661nm (λmax of MB+ ) using Genesys 10 UV-VIS Scanning Spectrophotometer. The reaction mixtures were allowed to undergo complete reaction as evident from the constancy of repeated measurements of absorbance (A∞) at 661 nm. The concentration at any time t (Ct) was obtained from the difference in the absorbance at time t (At) and at infinity (A∞) .
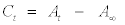 (1)
(1)
The efficiency of MB+ decolourization was calculated by using Equation 2:
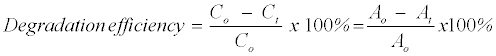 (2)
(2)
Where, Co and Ct are MB+ concentration at initial and any time respectively. The parameters Ao and At are the absorbance of the MB+ solutions in the 661nm wavelength at initial and any time respectively.
The effects of transition metal ions was investigated by adding various concentrations of transition metal solutions (COCl2.6H2O, NiCl2 .6H2O, CuSO4.5H2O, ZnCl2, FeSO4.7H2O and MnCl2.4H2O) in the range 0.1 – 0.5 mg/dm3 at 30°C with fixed amounts of MB+ (20 mg/ dm3) and Na2SO3 (1.0 mol dm-3).
Effect of initial concentration of MB+
The extent of MB+ decolorization by Na2SO3 with time at varying initial MB+ concentrations (20–70 mg/dm3) under identical conditions is shown in Figure 1. It is seen that the decolorization of MB+ is relatively fast at the beginning and decreases as the reaction progresses. At low concentration (20 mg/dm3), MB+ is almost completely decolorized by Na2SO3 within 60 s., which is also evident from the absorbance data recorded with reaction time (Figure 1a). The time profiles of MB decolorization show that the decolorization is substantially decreased with increase in initial MB+ concentration. For example, the extent of decolorization after 60 s decreases from 85% to 29% with increase in concentration of MB+ from 20 to 70 mg/dm3 with Na2SO3.
Effect of initial concentration of 2 SO32−
The effect of Na2SO3 on the decolorization of MB+ was investigated by varying initial concentration of Na2SO3 from 0.4 to 1.5 mol dm-3, the results are shown in Figure 2. It was observed that the decolourization efficiency increases from 69.6% to 80.9% as a consequence of increasing Na2SO3 concentration from 0.4 to 1.5 mol/dm3 within 60 sec. This is due to increasing reductive power of the sulphite. The maximum decolourization efficiency of about 99.4% was achieved with 1.5 mol/ dm3 of Na2SO3 within 5 min.
Kinetic study
The reductive decolorization of MB+ by sulphite was a complicated process probably due to various reduced forms of MB+ [22]. The process could not easily be depicted by simple reaction kinetics. To estimate the kinetics rate of decolorization, an equation form of power law is used:
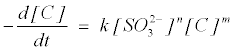 (3)
(3)
where k is the removal rate constant, [C] is the MB+ concentration, m and n are the pseudo order of reaction with respect to MB+ and SO32− respectively. The rate equation can be stated with observed rate constant (kobs) as follows:
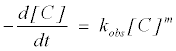 (4)
(4)
where,
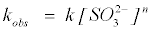 (5)
(5)
For a zero-order reaction, the above equation after integration becomes:
Ct = k0t(6)
Also for a first-order reaction, the above equation after integration becomes:
In (Ct) = In (C0) − k1t(7)
In which, Co is the initial MB+ concentration and Ct is the concentration at reaction time t. For a second-order reaction, the integrated equation becomes:
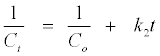 (8)
(8)
The absorbance-time data plot of the above reaction orders were carried out to determine the rate constant, kobs and best order for the reaction process. The zero-, first- and second-order kinetics rate constants for the decolorization of MB+ with SO32− were obtained from the plots, and the results were shown in Table 2. It was observed that the correlation coefficients for the three models are different. The firstorder kinetics shows highest correlation (R2>0.9) than both zero and second-order kinetics (R2<0.9). It can therefore be concluded that the decolorization of MB+ with SO32− fits the first-order reaction kinetics of the type:
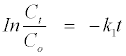 (9)
(9)
From the pseudo first-order kinetic plots (Figures 3a and 3b), the pseudo-first order rate constants with respect to MB+ and SO32− were obtained (Table 1).
| [SO32–], moldm–3 | Zero order | First order | Second order | ||||
|---|---|---|---|---|---|---|---|
| ko x 10-3 | R2 | k1 x 10-3 | R2 | k2 x 10-2 | R2 | ||
| 0.4 0.6 0.8 1.0 1.2 1.5 |
1.6 1.8 2.4 2.4 3.0 3.1 |
0.576 0.547 0.554 0.422 0.457 0.485 |
8.0 8.6 11.1 12.8 14.8 15.2 |
0.960 0.975 0.972 0.968 0.966 0.978 |
9.8 15.0 16.1 24.6 27.8 28.1 |
0.506 0.625 0.598 0.704 0.659 0.678 |
|
| [MB+] mg/L | Zero order | First order | Second order | ||||
| ko x 10-3 | R2 | k1 x 10-2 | R2 | k2 x 10-1 | R2 | ||
| 20 30 40 50 60 70 |
3.6 3.6 3.8 4.2 4.5 3.3 |
0.452 0.634 0.764 0.802 0.832 0.890 |
1.67 1.48 1.28 1.40 1.38 0.86 |
0.968 0.972 0.993 0.978 0.972 0.962 |
2.81 2.78 1.73 1.96 1.89 0.81 |
0.705 0.629 0.550 0.528 0.533 0.449 |
|
Table 1: Kinetics rates of reaction with respect to MB+ and SO32− .
The pseudo first-order rate constant decreased with increasing initial concentration of methylene blue (Figure 4a). The possible explanation is that at high dye concentrations, the reductive activity of sulphite might be reduced due to coverage of reducing ion species by the dye ions.
Similarly, the pseudo first-order rate constant increased with increasing concentration of SO32− . The explanation for this trend is that at high sulphite concentration, there is high concentration of reducing ion species which override the coverage effect of the dye ions. The plot of kobs versus sulphite concentration ranging from 0.4-1.5 mol dm-3 shows a linear relationship between kobs and [SSO32−] (Figure 4b). This fact reveals also first-order dependence on the reductant. An overall rate equation can then be explained in the form:
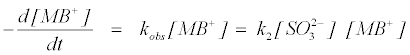 (10)
(10)
where k2 is the overall second order rate constant.
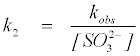 (11)
(11)
Effect of pH
In view of the fact that pH of dye solution is a main parameter on the decolorization progress, the experiments were carried out to find the optimal pH of reaction mixture for decolorization of MB+. The examined pH range was from 1-8, which was adjusted by using appropriate amounts of base (NaOH) or acid (HCl) solutions. It was observed that the decolorization of MB+ was significantly influenced by the solution’s pH values, and the highest decolorization rate (kobs) was achieved at pH 1.45. These observations are in good agreement with published results [40,53-56].
The plot of k2 versus [H+] was linear with a positive intercept. The acid dependent rate equations for the reactions can be represented by equation 12.
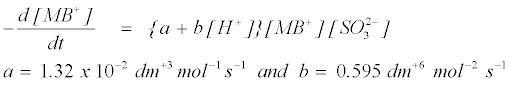 (12)
(12)
The above equation (12) suggests that the reaction occurs via aciddependent and acid-independent pathways. The high rate constant value observed at lower pH can be explained by the change in the MB+ structure. The labile H atom makes the molecule of MB+ dye vulnerable towards the attack of reductant. This has been explained in terms of protonation of SO32− in a fast step to give HSO3-which subsequently reacts with the substrate in a slow step to give the product [57].
Effect of transition metal ions
Different transition metal ions, Fe(II), Cu(II), Ni(II), Mn(II), Zn(II) and Co(II) were tested as a catalyst for the decolorization of MB+ with SO32− . The results show that Fe(II) and Cu(II) accelerate the reaction rate with varying catalytic activity (Figure 6), while others have no catalytic effect on the rate of reaction (Table 2). It was observed that the rate of reaction between methylene blue and sodium sulphite was increased by the presence of trace amounts of Fe (II) and Cu(II) ions. A plot of rate constant versus [Fe (II)] and [Cu (II)] gives a straight line with almost the same values of intercept of about 2.0 s–1, which is equal to the rate of the uncatalyzed reaction. The values of slopes are quite different: 7.68 and 2.48 dm+3 mg–1 s–1 for Fe(II) and Cu(II) respectively.
| [M2+]/ mg dm–3 | kobs x 10–2/ s–1 | |||||
|---|---|---|---|---|---|---|
| Cu2+ | Fe2+ | Zn2+ | Mn2+ | Co2+ | Ni2+ | |
| 0.1 0.2 0.3 0.4 0.5 |
1.03 1.05 1.12 1.20 1.45 |
2.94 4.40 4.54 5.04 6.46 |
1.24 1.28 1.55 1.51 1.51 |
1.32 1.42 1.43 1.51 1.45 |
1.37 1.45 1.39 1.46 1.11 |
0.63 0.95 1.08 0.89 1.04 |
Table 2: Influence of transition metal ions on the rate of decolorization (kobs) of MB+ with SO32− . ([MB+]o = 20 mg/dm3; [Na2SO3] = 1.0 mol/dm3; pH = 8-9; T = 30°C ).
The rate constant in the presence of Fe2+ and Cu2+ ions is greater than in the absence of these transition metal ions. This could be due to activation of sodium sulphite by these transition metal ions to produce sulphate anion radical (SO4•− ) via sulfite radical anion [58-60]. The sulfite can be oxidized to sulphate by trace transition metal ions via free radicals. This metal-catalyzed autoxidation generates sulfur trioxide anion radical (SO3•− ) in an initiating one-electron oxidation step through an oxygen-consuming chain reaction [61-64] in accordance with the following reaction scheme 2.
Effect of temperature
The effect of temperature on decolorization of methylene blue in the presence and absence of transition metal ions catalyst was investigated in the range of 293-313 K. The results in Figure 7 show that the decolorization efficiency of methelene blue gradually increased with increase in the temperature. The increase in temperature may lead to increasing reduction rate of methylene blue.
The activation parameters associated with the decolorization of methylene blue are calculated from the plot In kobs versus 1/ T (Figure 7), which gives the value of activation energy (Ea) , according to the Arrhenius equation:
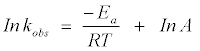 (13)
(13)
The values of ΔH# and ΔS# can be calculated from the plot of the Erying equation [65] as follows:
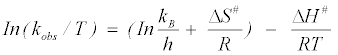 (14)
(14)
Where, kB is the Boltzman’s constant (1.381×10–23 J K–1); h is the Plank’s constant (6.626×10–34 J.s), where 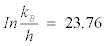
From the linear plot of 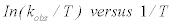 (Figure 7), values of ΔH# and ΔS# can be calculated. The Gibbs’ free energy ΔG# can therefore be calculated from the relation:
(Figure 7), values of ΔH# and ΔS# can be calculated. The Gibbs’ free energy ΔG# can therefore be calculated from the relation:
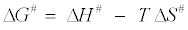 (15)
(15)
The rate constants and activation energies in the range of temperature studied (20-40°C) are listed in Table 3 while other thermodynamic parameters are given in Table 4. The positive values of ΔG# for the reaction (Table 4) indicate the non-spontaneous nature of decolorization of MB+ by sulphite at the temperatures studied. The negative value of ΔS# suggest the decreased randomness at the liquid/ liquid solution interface during the decolorization of MB+ solution by sulphite ion while the positive values of ΔH# show the endothermic nature of the reaction.
| T (°C) | kobs x 10–2 / s–1 | Ea / kJ mol–1 | ||||
|---|---|---|---|---|---|---|
| Absent | Fe2+ | Cu2+ | Absent | Fe2+ | Cu2+ | |
| 20 25 30 35 40 |
0.83 1.11 1.67 1.89 2.13 |
2.14 3.92 4.54 5.24 6.11 |
1.89 2.15 2.91 3.44 4.32 |
38.13 | 37.42 | 33.33 |
Table 3: Rate constants and activation parameters for interaction of MB+ and SO32− in the presence of catalyst. ([MB+]=20 mg/L; [ SO32− ] = 1.0 mol/dm3; [Catalyst] = 0.3 mg/dm3; pH = 8-9; T = 30°C).
| M2+ | ΔH# / kJ mol−1 | ΔS# / kJ mol−1K−1 | ΔG#298K/ kJ mol−1 |
|---|---|---|---|
| Absent Fe2+ Cu2+ |
35.56 34.83 30.78 |
– 0.162 – 0.156 – 0.173 |
84.13 81.32 82.33 |
Table 4: Thermodynamic parameters for the reaction between MB+ and SO32− . ([MB+]=20 mg/dm3; [ SO32− ]=1.0 mol/dm3; [Catalyst]=0.3 mg/dm3; pH = 8-9; T = 30°C).
The activation energy in presence of Fe(II) and Cu(II) is lower than in its absence (Table 3). The decrease in the activation energy (Ea) in presence of these transition metal ions confirms their catalytic effects (metal activation).
Reaction mechanism
Based on the above experimental findings and observations, the reaction mechanism has been suggested utilizing the redox properties of Na2SO3. It has also been noted that redox reactions of many oxyanions are strongly acid dependent [66]. Under the present experimental conditions, it is reasonable to postulate that SO32− is protonated in a fast step to give HSO3− which then reacts with MB+ in a slow step to give the products [56]. Also, the intercept obtained from the plot of k2 versus [H+] (Figure 5) indicates that the unprotonated SO32- also reacts with MB+ to form the products. Therefore, taking recourse to the experimental data, the following mechanistic steps have been postulated for the reaction:
 (16)
(16)
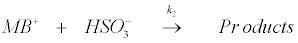 (17)
(17)
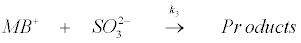 (18)
(18)
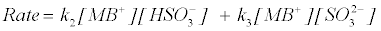 (19)
(19)
From equation (16)
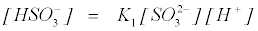 (20)
(20)
Putting equation (20) into (19);
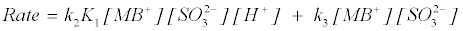 (21)
(21)
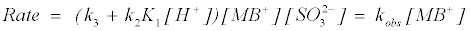 (22)
(22)
where K1 is the protonation constant, and k2 and k3 are the pseudosecond order rate constants for the protonation and deprotonation of MB+. Equation (22) shows acid dependence on the rate of decolorization of methylene blue with sodium sulphite and is similar to equation (12) with k3=‘a’=1.32×10–2 dm+3 mol–1 s–1 and k2K1=‘b’=0.595 dm+6 mol–2 s–1.
The color removal of the cationic dye methylene blue by the formation of a reduced form of MB+ (colorless) is studied kinetically. The rate of color removal depends on the concentration of reactants, pH, and temperature. The decolorization of MB+ is pseudo first order with respect to MB+ and SO32− . The decolorization increases with temperature, in acidic medium, after addition of Fe(II) and Cu(II), but decreases with the addition of Mn (II), Ni(II), Co(II) and Zn(II).