Journal of Physical Chemistry & Biophysics
Open Access
ISSN: 2161-0398
ISSN: 2161-0398
Research Article - (2016) Volume 6, Issue 3
The kinetics and mechanism of an eco-benign clay catalyst employed in the production of ethyl acetate was evaluated. The results obtained revealed that the conversion of acetic acid was dependent on the catalyst weight, reaction time and mole ratio. The maximum conversion of acetic acid was obtained for mole ratio (acid: alcohol) of 2:1 with optimum catalyst weight of 2.0g at a reaction temperature of 363K and 150 minutes time on-stream. Kinetic studies revealed that the esterification reaction was second-order and followed the single step Eley-Rideal reaction mechanism.
<Keywords: Al-Pillaring, Clay catalyst, Esterification, Kinetics and mechanism
Esterification reaction generally refers to the formation of esters by the interaction of alcohols and carboxylic acids. Alternatively, it refers to as the process of formation of an ester by the reaction between an alkanol and an acid. It is a reversible process and does not proceed to any appreciable extent in the absence of catalysts or supercritical condition [1]. This process is described as an acidcatalyzed equilibrium synthesis developed by Emil Fischer. It is the simplest pathway among several pathways used in synthesis of esters. When catalysed by a strong acid usually tetraoxosulphate (VI) acid, the reaction is called Fisher esterification [2]. This important process is one of the most organic reactions in chemical and allied industries visa vis its applications as intermediate in the synthesis of fine chemicals, drugs, perfumes, food preservatives, and also in the production of biodiesels via transesterification. It is widely applied from the preparation of highly specialized esters in the chemical laboratory to the production of millions of tons of commercial ester products [3]. Esterification process can be carried out either as a batch or a continuous process. The batch procedure involves a single pot reactor that is filled with the acid and alcohol reactants. The acid catalyst is added and the water removed as the reaction proceeds. This method is most often used in the chemical laboratories, but in a few cases, it is used by industry to make large quantities of esters [4]. Ethyl acetate is a colorless liquid with a characteristic smell. It has wide applications especially as solvents used by many industrial, beverage and pharmaceutical outlets [3]. In order to eliminate the corrosiveness and deterioration of the reaction plants and environment by mineral acids used as catalyst, the esterification of carboxylic acids with alcohols in the presence of solid acid catalysts that are eco-friendly has gained the attention of many researchers in recent times. From academic standpoint, the mechanism proposed for the esterification reaction involving an alcohol and carboxylic acid is an acid promoted acyl substitution, which results in the substitution of an alkoxy group for the hydroxyl portion of the carboxyl group. In this reaction, a carboxylic acid does not react with an alcohol unless an acid catalyst is used; protonation makes the carbonyl group more electrophilic and enables it to react with the alcohol, which is a weak nucleophile [5]. Clays and clay minerals are solid acid green chemical catalysts according to Kurian and Kavitha, [6] which can function as both Bronsted and Lewis acids in their natural and modified states. The clay mineral used in this study is “Bentonite”. Bentonite clay is one of the most abundant smectitic clays in nature. In Nigeria, bentonite deposits had been found in different parts of the country. An estimated reserve of about 700 million tones had been indicated in the North Eastern (Black cotton soil) part of Nigeria. These areas comprise of Borno, Yola, Adamawa and Taraba states [7]. There are also commercial quantity deposits of bentonite in the Southern part of Nigeria. This large bentonite reserve awaits commercial exploration for its various applications. Pillared interlayer clays (PILCs) are an interesting class of two-dimensional micro-porous and meso-porous materials [8]. The need to transform natural clay to pillared forms is to improve on its surface area and porosity. The pillared clays are very attractive solids for adsorption and catalysis purposes [9]. Therefore, this work looks at the kinetics and mechanism of the application of an eco-benign solid catalyst (Al-PILC) in the synthesis of ethylacetate (ester).
Natural clay sample was collected from the open clay deposit in Ezinachi, Okigwe Local Government Area, Imo state, Nigeria. The clay sample was washed and sun-dried for two days. Aluminum pillared material was produced by ion exchange and calcinations at 473 K. Esterification reactions were carried out in a batch mode using a three-necked round bottom glass flask of 250 ml capacity fitted with a reflux condenser and mercury in glass thermometer to monitor the temperature. Heating and stirring was achieved using a magnetic hot plate with a stirrer. Acetic acid and the clay catalyst (for 1:1, 2:1, 3:1, 4:1 acid: alcohol mole ratio) were charged into the reactor and heated to 363 K. After the desired temperature has been reached, a known amount of ethanol preheated separately using heating mantle was added into the reactor. The kinetic measurement was done by varying the time of the process from 0-150 mins. The reaction mixture (2 ml) was taken immediately using Pasteur pipette and titrated against 0.1 M NaOH solution using phenolphthalein indicator. All the experimental runs were designed by varying the amount of the catalyst, the acid to alcohol mole ratios, and the reaction period while keeping the temperature constant for all the runs. The concentration of acetic acid consumed during the reaction was calculated by the following formula below [10].
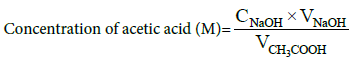 (1)
(1)
Where CNaOH=Concentration of sodium hydroxide in molarity, VNaOH=Volume of sodium hydroxide used in titration in dm3 and VCH3COOH =Volume of reaction mixture sample titrated measured in dm3.
However, the percentage conversion of acetic acid was equally calculated by the formula below [11]:
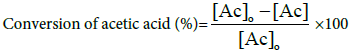 (2)
(2)
Where [Ac]o=initial concentration of acetic acid (M) and [Ac]=measured concentration of acetic acid at time of sampling (M).
Kinetics of esterification of acetic acid and ethanol
The reaction kinetics for the esterification of acetic acid and ethanol using pillared clay catalysts was analysed by using the integral method of analysis of rate data to determine the order of the esterification reaction [12]. In order to determine the order of the esterification reaction, the equations 3 and 4 below were used to plot a graph of In[CH3COOH]t against time t and 1/[CH3COOH]t against time t.
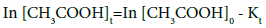 (3)
(3)
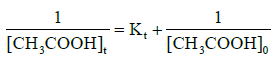 (4)
(4)
Where [CH3COOH]o and [CH3COOH]t are the concentration of acetic acid at zero time and at a particular time t respectively. Equations 3 and 4 are for first and second-order reactions. The plots of first-order reaction for Al-pillared clay as regards to loading and mole ratio are represented in Figures 1 and 2 while plots of second-order reaction for Al-pillared clay are represented in Figures 3 and 4. From the plots, it was observed that the correlation coefficient (R2) of the second-order reaction gave best fit compared to the first-order reaction, hence it can be concluded that the esterification of acetic acid and ethanol using Alpillared clay catalyst obey the second-order reaction equation. The rate constants obtained are presented in Tables 1-3.
| Acid to alcohol mole ratio | Rate constant K (dm3mol-1s-1) |
|---|---|
| 1:1 | 0.0059 |
| 2:1 | 0.0040 |
| 3:1 | 0.0013 |
| 4:1 | 0.0006 |
Table 1: Reaction rate constants for formation of ethyl acetate for various acid: alcohol mole ratios using 2.0 g of Al-pillared clay catalyst at 363 K.
| Catalyst weights (g) | Rate constant K (dm3mol-1s-1) |
|---|---|
| 0.5 | 0.0019 |
| 1.0 | 0.0025 |
| 1.5 | 0.0034 |
| 2.0 | 0.0040 |
Table 2: Reaction rate constants for formation of ethyl acetate for various catalyst weights of Al-pillared clay at 363 K and acid: alcohol mole ratio of 2:1.
| (CA, o) (M) | 1/CA, o(1/M) | (Ro) (M/min) | 1/Ro(min/M) |
|---|---|---|---|
| 1.05 | 0.95 | 0.01 | 85.71 |
| 0.96 | 1.05 | 0.003 | 315.76 |
| 0.87 | 1.16 | 0.003 | 333.33 |
| 0.79 | 1.27 | 0.0025 | 400.00 |
| 0.75 | 1.34 | 0.0015 | 666.67 |
Table 3: Initial reaction rate values for the mechanistic pathway.
Reaction mechanism
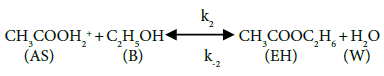
In this study, the reaction mechanism according to Fogler [13] can be written as follows:
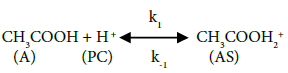 (5)
(5)
 (6)
(6)
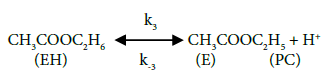 (7)
(7)
The above mechanism, equations 5-6 depicts the activation of carbonyl oxygen of acetic acid by the pillared clay catalysts, followed by the nucleophilic attack of protonated acetic acid by ethanol forming ester and water, and the deprotonation of ethyl acetate respectively.
The rate equation for the first step of the mechanism (equation 5), can be written as:
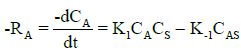 (8)
(8)
Under steady-state conditions, the rate of production of intermediates (AS, EH) can be equated to zero. Thus, rates for (AH) and (EH) becomes:
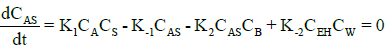 (9)
(9)
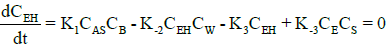 (10)
(10)
Since ethanol is a strong nucleophile, it competes for the catalyst acid sites with acetic acid as does water once it is produced from reaction.
Considering the initial reaction period when reverse hydrolysis is not important. Using the pseudo-steady state approximation equation for the intermediates (AS, EH) and solving for CAS with the site balance (ρ.CC=CAS+CBS+CS) gives the rate expression as:
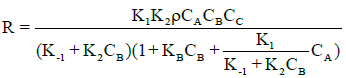 (11)
(11)
Where CC, CB, CA, and CS are the concentrations of catalyst, ethanol, acetic acid and vacant acid site on the catalyst surface, respectively; ρ denotes the site density of the catalyst, CBS is the concentration of ethanol adsorbed on the catalytic acid sites, kB represents the adsorption equilibrium constant for ethanol on the acid sites; k1 and k−1 are the acetic acid adsorption and desorption constants, respectively; and k2 is the surface reaction constant.
If the surface reaction (k2) is favoured over the adsorption and desorption of the acid (k1 and k−1), 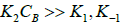 , then equation (11) can be approximated as:
, then equation (11) can be approximated as:
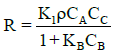 (12)
(12)
If, on the other hand, the opposite is true with the adsorption and desorption process being faster than surface reaction, k2CB « k1, k-1, then equation (11) reduces to:
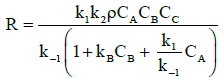 (13)
(13)
Defining kA=k1/k-1 as the adsorption equilibrium constant of the acetic acid, we have
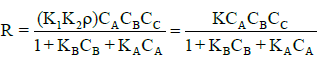 (14)
(14)
Where k is equivalent to kAk2ρ. Using equation (14) and initial reaction rate (Ro) measured at various initial acid concentrations; a mathematical model was developed to predict esterification mechanism.
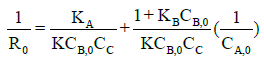 (15)
(15)
For a plot of 1/Ro against concentration of acid at fixed alcohol concentration, if the plot pass through a maximum then it means that the esterification reaction proceeds through a Langmuir-Hinshelwood (LH) mechanism, but if there is no such maximum encountered, it means that the esterification reaction follows an Eley-Rideal (ER) mechanism [14]. From the plots of 1/Ro against 1/CA,O for Al-pillared catalyst represented Figures 5 and 6, it showed that the initial reaction rate increases linearly with acid concentration, thus suggesting that the esterification of acetic acid and ethanol follow ER mechanism [3].
The kinetics and mechanism has shown that the esterification of acetic acid and ethanol followed second-order reaction equation and Eley-Rideal (ER) mechanism. This work has demonstrated that Al-pillared clay catalyst has potential for esterification and transesterification of carboxylic acids into important products.
The Authors are grateful to Cardiff Catalysis Institute, School of Chemistry and University of Cardiff, United Kingdom.
Authors have declared that no competing interests exist.