Journal of Clinical and Experimental Ophthalmology
Open Access
ISSN: 2155-9570
ISSN: 2155-9570
Review Article - (2021)
Kyrieleis plaques represent focal segmental retinal arteritis or periarteritis and are usually associated with infectious posterior uveitis or non-infectious autoimmune vasculitis. This review showcases the possible infectious and noninfectious associations, pathogenesis, clinical presentation, differential diagnosis and multimodal imaging of Kyrieleis vascular plaques. MedLine and PubMed search was performed pertaining to Kyrieleis plaques, segmental retinal arteritis and periarteritis, pathogenesis of Kyrieleis plaques, Fluorescein angiogram of Kyrieleis plaques, Indocyanine green angiogram of Kyrieleis plaques Optical coherence tomography of Kyrieleis plaques, multimodal imaging of Kyrieleis plaques.
Kyrieleis plaques; Fundus fluorescein angiography; Indocyanine angiography; Optical coherence tomography
Kyrieleis plaques is referred by several names in the literature as Kyrieleis vasculitis, Kyrieleis retinal arteriolitis, segmental retinal periarteritis and nodular periarteritis [1].
Kyrieleis plaques were first described by Werner Kyrieleis in 1933 as ring-like exudates extending along the width of the arteries and particularly where the vessels divided. The veins were not affected and he hypothesized that these plaques may have been following an allergic reaction to the tubercle bacilli or its toxins [2]. In the past, retinal periarteritis was associated only with tuberculosis [2] or as a focal reaction to the tuberculin test [3], but in the years that followed there were reports of segmental retinal periarteritis in those without tuberculosis and that showed remarkable response to corticosteroids [4]. Other associations were Toxoplasma gondii [5,6] Treponema pallidum [7,8] Cytomegalovirus [9], Herpes Zoster virus associated with acute retinal necrosis [10], Varicella Zoster [11,12], Herpes Simplex [13], Rickettsia conorii [14], intra ocular lymphoma [15], arterial macro aneurysms [16], and collagen vascular disease [17]. Toxoplasma gondii has been the most common association of Kyrieleis plaques. Among the non-infectious causes, Kyrieleis plaques have been described in association with auto immune Behcet’s disease [6]. Kyrieleis plaques have been seen in association with recurrent episodes of multiple Branch Retinal Artery Occlusions (BRAO) [18,19] and Susac Syndrome (recurrent BRAO, tinnitus, hearing loss and encephalopathy) [20,21].
Griffin and Bodian conjectured that these plaques may have been caused by migration of exudates from active choroiditis lesions to the periarterial sheaths. The segmental nature of the lesion was speculated to be due to the arrangement of the perivascular sheaths into compartments or as a result of inflammatory allergic reaction similar to hives [4]. Orzalesi and Ricciardi hypothesized that theses plaques may have been a result of immune reaction to bacterial toxins or sometimes drugs with deposition of immune cells and inflammatory debris within the retinal arterial walls [22]. Wise, on the other hand speculated arteriosclerosis as the probable pathogenesis [23]. Pichi and colleagues proposed that these plaques were as a result of severe intraocular inflammation and endothelial inflammation (within the vessel wall) indicating endothelitis and not periarteritis as had been speculated earlier [24].
Gass et al. speculated that in those with recurrent BRAO, there were no visible emboli or any source of emboli and the plaques may have been due to the immune complexes in the arterial walls that led to focal arteritis and arteriolitis. These plaques that are seen along the obstructed arterial segment can remain permanently too [18,19].
Gass et al. hypothesized that in Susac syndrome these plaques occurred at mid arterial segments away from the bifurcations and may have been due to localized immune-mediated reaction in the arterial walls which were similar to that occurred in the brain or inner ear [19-21].
In non-Hodgkins large cell lymphoma, the theory claims that the plaques are nothing but non-obstructive atheromatous plaques consisting of tumour infiltration and lipid-laden macrophages at the site of arterial damage. The lymphomatous infiltration may have triggered the formation of lipid atheroma with deposition of lipid-laden macrophages following damage to the endothelium. The atheroma may be a possible pathogenesis in the recurrent BRAO group too, as they do not demonstrate any active area of choroidal inflammation [15,16].
Huang et al. reported a case of retinal artery macroaneurysm (RAM) following Kyrieleis arteriolitis in a patient with toxoplasma vasculitis. The proposed mechanism was that vasculitis and Kyrieleis arteriolitis may have led to the formation of emboli and subsequent RAM [25].
Clinical diagnosis
Kyrieleis plaques present as yellowish-white deposits along the vessels particularly, arteries in a segmental manner. They always occur besides an area of active chorioretinitis near the arteries and in association with severe intraocular inflammation. They involve the retinal arteries only [6,13,24]. Sometimes, these plaques can become apparent after the inflammation subsides [1,26]. Kyrieleis plaques have a glistening appearance and resemble calcific lesions and involve the arteries only [6,24].
Multimodal imaging
Fluorescein Angiography (FA): FA shows hypo fluorescence of the plaques in the early phase and increasing hyper fluorescence in the late phase. There was no occlusion of vessel and no leakage of dye and the plaques were confined to retinal arteries only. Few authors have shown the FA to be normal throughout all the phases of angiogram. There was no delay in the arterial filling and there may be leakage and staining from the retinal veins [6,7,22,24].
FA in those with recurrent BRAO may show leakage from the arterioles in few areas with the majority of them showing normal perfusion and no abnormal angiographic findings [16,18] . FA in those with Susac syndrome did not show any occlusion in the area of the arterial plaques [21].
Indocyanine Green Angiography (ICG): ICG showed features exclusive for Kyrieleis plaques. ICG showed well demarcated, dense and early hyper fluorescence of the vessel walls which persisted in the late phases too. ICG is able to bind to the inflammatory proteins that constitute the plaques because of its amphiphilic nature and thus produce hyper fluorescence that lasts through all phases. To take up stain by ICG, the plaques should come in contact with the dye and ICG does not show any filling defect which further confirms the fact that the plaques are within the vessel wall and not outside [24].
Fundus Auto-Fluorescence (FAF): FAF shows hyper auto- fluorescence of the plaques and correlate with the focal inflammation seen in the endothelium of the vessels. This has been shown to be similar to that seen in the other blood vessels like coronary arteries and aorta [24].
Optical Coherence Tomography (OCT): Spectral Domain OCT (SD OCT) shows intramural hyperreflective deposits in the acute stage corresponding to the plaques. The hyperreflectivity is of the vessel wall with a normal reflective lumen [24]. SD- OCT has also demonstrated a pre retinal hyper reflective spot, presumably a Kyrieleis deposit in the foveal area which persisted for several weeks and then disappeared. There was no associated cystoid macular edema or any long-term sequelae [28,29].
Kyrieleis plaques were first described by Werner Kyrieleis with regard to tubercular uveitis and sofar only very few cases have been reported in the literature. There have been no pathological studies or histological examination done to throw light upon the etiology or the mechanism involved in the development of these plaques.
Segmental periarteritis is usually a secondary finding associated with severe inflammation. These plaques are presented adjacent to an area of chorioretinitis near the involved arteries [24] (Figures 1a-1d).
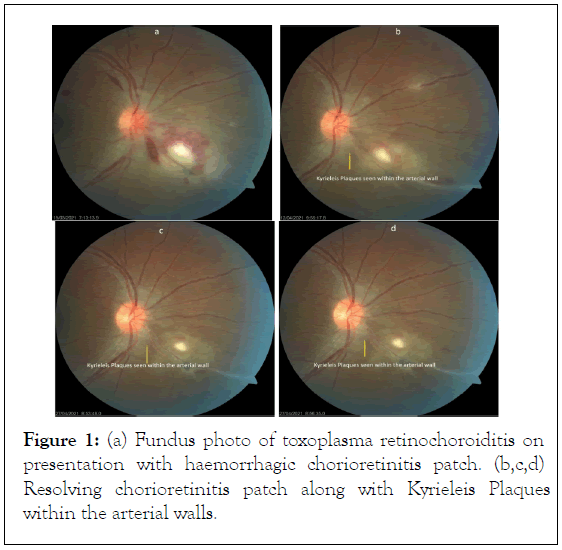
Figure 1: (a) Fundus photo of toxoplasma retinochoroiditis on presentation with haemorrhagic chorioretinitis patch. (b,c,d) Resolving chorioretinitis patch along with Kyrieleis Plaques within the arterial walls.
They may go unnoticed initially because of severe inflammation only to be apparent once the inflammation subsides[26] and the chorioretinitis patch heals [22]. These plaques can either increase in number [10] or disappear after few weeks [22] or remain indefinitely even after the lesion resolves and the inflammation subsides [7].
There are various mechanisms that have been hypothesized but no definitive theories have been put forward regarding the possible pathogenesis of Kyrieleis plaques. These plaques could have been due to migration of exudates to the periarterial sheaths from an area of active chorioretinitis or as a result of an immune reaction in response to drugs or bacterial toxins [22] or arteriosclerosis [23].
The deposition of immune complexes causing arteritis or arteriolitis with no visible emboli has been speculated in cases of recurrent BRAO [18,19].
The recent hypothesis however states that it is endothelitis and not periarteritis as was previously thought of [24]. In cases where there is no associated inflammation, a theory of atheromatous plaques containing lipid atheroma has been proposed.
This was evident in a patient with lymphoma wherein the lymphomatous infiltration may have damaged the vessel endothelium that caused lipid transfer into the arterial walls [15,16]. However, none of these theories could explain the reason for the segmental involvement of the vessels.
Earlier, they were associated with tubercular uveitis [2], and then these plaques were frequently seen in association with toxoplasma retinochoroiditis [5,6]. The associations in toxoplasmosis may have been due to the increased frequency of toxoplasma uveitis.
When a question of whether these plaques are seen only in immunocompetent individuals arose, there were reports of these plaques in immunocompromised individuals too as in a HIV-positive patient with syphilitic uveitis confirming that it is not pathognomonic of only toxoplasmosis or immunocompetent individuals [8]. Bilateral plaques have been reported in the literature and are determined by the underlying etiology [11,12,29].
Kyrieleis plaques are whitish-yellow glistening deposits seen along the outer walls of retinal arterioles with high reflectivity. The high reflectivity of the vessel walls may confuse this with sheathing. They are not intraluminal or endothelial deposits and this differentiates it from arterial emboli which are intraluminal [6,24].
They are present in the same quadrant as the retinochoroiditis lesion in those with toxoplasma. These are segmental and may be associated with sheathing of the neighbouring vessels and are concentrated at the arterial branchings [24,26].
FA findings showed no occlusions or leakage in the area of the plaques and no delay in the retinal arterial filling. Also, FA of the arteries was reported to be normal with leakage from the retinal veins. Hyperfluorescence in the area of retinitis were observed particularly in those with toxoplasma retinochoroiditis (Figures 2a-2d) .
Figure 2: (a) Fundus fluorescein angiography of toxoplasma retinochoroiditis shows masked fluorescence in the area of haemorrhage and chorioretinitis in the initial phases. Segmentation of veins is seen in the early phases. Increase in intensity is seen in the area of chorioretinitis patch toward the late phases. (b, c, d) Perivascular cuffing with increasing intensity is seen above the disc with disc hyperflourescence.
There were no occlusions at the site of these plaques in those with recurrent BRAO or Susac syndrome. Thus, FA plays a very crucial role in differentiating this from retinal emboli which cause filling defect. FA also helps in differentiating these plaques from frosted branch angiitis which involve both arteries and veins and profusely leak fluorescein, while Kyrieleis plaques are arterial and do not leak fluorescein [24,26].
ICG staining of the inflammatory lesions is explained by the amphiphilic nature of ICG in contrast to fluorescein which is hydrophilic. The hyperfluorescent nature and staining of the vessel wall further clarifies that the lesions are located within the vessel wall [24]. This can be compared to the candle wax drippings in sarcoidosis and frosted branch angiitis wherein they are located outside the vessel wall and do not take up stain but showing only masking of the underlying fluorescence [24,30,31]. The damaged endothelium attracts inflammatory macromolecules without damaging the vessel wall and hence there is no leakage. The fibrin from the damaged lumen might account for the delayed and long-standing fluorescence as seen in cases of central serous retinopathy [24,32].
SD-OCT has confirmed the location being within the arteries and not outside the vessel wall. The region of the plaques is demonstrated by the hyper reflectance of the vessel wall corresponding to the areas of hyper fluorescence seen on ICG. This confirms that the inflammation is within the vessel wall with no intraluminal deposits in contrast to frosted branch angiitis where the hyper reflectance with hyper fluorescent spots is seen in the perivascular area [24,31].
Hyper autofluorescence of these plaques have been reported during the course of the disease at the site of endothelial
inflammation and might be due to the deposition of some secondary calcified material that is different from lipofuscin [24].
Kyrieleis plaques are benign lesions which may disappear or increase in number temporarily or persist indefinitely. The pathogenesis of Kyrieleis plaques remains a mystery till date. Various theories have been speculated however, there has not been any lucid information regarding the mechanism involved in the development of these plaques. The glistening appearance of Kyrieleis plaques may be clinically difficult to differentiate from other retinal vasculitic lesions where both arteries and veins are involved, but the exclusive arterial and endothelial involvement along with absence of leakage or obstruction of lumen or retinal arterial non-perfusion in FA will help differentiate this from other conditions.
Citation: Priya RC (2021) Kyrieleis Plaques: A Review. J Clin Exp Ophthalmol. S18:002.
Received: 10-Aug-2021 Accepted: 24-Aug-2021 Published: 30-Aug-2021 , DOI: 10.35248/2155-9570.21.s18.002
Copyright: © 2021 Priya RC. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.