Pediatrics & Therapeutics
Open Access
ISSN: 2161-0665
ISSN: 2161-0665
Research Article - (2021)Volume 11, Issue 7
Background: Urinary tract infections (UTIs) are a common and potentially serious bacterial infection of childhood. UTI is the most common cause of hospitalization in children with high fever especially in infancy, which is more common to be the source for bacteremia in this age. In infancy, the disease is more common in boys and the incidence in girls increases with age, it has been found that uncircumcised children have a higher incidence of urinary tract infection. 30% of children who have had the first infection of pyelonephritis (inflammation of the kidney tissue) will develop a recurrent urinary tract infection when the infection occurs in the first year of life. The most common bacteria to cause these infections are Escherichia coli (E-coli), later Gram-negative bacteria (-) such as Klebsiella pneumonia and Proteus mirabilis, and gram-positive (+) bacteria such as Enterococci. ESBL + resistant bacteria have been found more as uropathogenic in recurrent urinary tract infections. Our aims in this study are to describe the epidemiological and microbiological properties and imaging of urinary tract infection in young children up to the age of five years. In our study, we examined the significance of reflux, its degree, and whether a cystography examination is necessary for any upper urinary tract infection. In addition, it was important to study different indices of inflammation (leukocytes, C-reactive protein test), renal ultrasound findings, and renal mapping of DMSA.
Research methods: The study is retrospective that included infants and children up to the age of five who were hospitalized at Ziv Medical Center Safed in northern Israel in the pediatric department between 2010-2019, with the diagnosis of the first episode of urinary tract infection or recurrent episodes in the first five years of life. All children had symptoms and signs of urinary tract infection. The children underwent laboratory tests including cell blood count (CBC), kidney and electrolyte functions, C-reactive protein (CRP), blood culture, and urine sample. Following imaging of renal and urinary tract ultrasound, cystography was done after 4-6 weeks according to the protocol. Children underwent renal mapping with DMSA after 4 to 5 months of acute urinary tract infection.
Results: The data showed a greater tendency for girls aged one year and older to have UTI. The average hospitalization time was 48 to 72 hours. We found no association with maternal smoking. Breastfed babies have lower rates of urinary tract infections in the first year. The most common uropathogens are E-coli, Klebsiella pneumonia, Proteus mirabilis, and Enterococci. Antibiotic resistance was observed in recurrent infections by E-Coli 20%, ESBL to 30%, And Klebsiella 45%. 30% to 40% developed recurrent urinary tract infections, recurrent infections were within two to four months after the first infection. In most children, empiric antibiotic therapy of Ampcilin and aminoglycoside was started. Most children underwent renal ultrasound, 35% had pathological imaging of hydronephrosis, and some with hydroureter. 40% of the children who underwent cystography had reflux with varying degrees, 60% of those who did mapping (DMSA) were pathological mapping (filling defect, scar). 18% of children with normal DMSA results had reflux. CRP, rates were found to be high in all children with pyelonephritis and reflux.
Conclusion: Initiating empirical treatment of urinary tract infection is very important in preventing renal scar development. Recurrent urinary tract infections are characterized by various uropathogenic bacteria and a tendency to antibiotic resistance. Therefore, it is necessary to choose the appropriate antibiotic. As a renal ultrasound imaging available examination without radiation can be performed in the hospital. Cystography is not required in most cases of upper urinary tract infection. Kidney mapping by DMSA can reveal the additional value of kidney damage. Inflammatory indices including erythrocyte sedimentation rate (ESR) and CRP indicate the presence of renal tissue involvement such as Pyelonephritis. Keywords: Urinary tract infection; Pyelon
Urinary tract infection; Pyelonephritis; Ultrasonography; Dimercaptosuccinic acid (DMSA); Vesiclouretral reflux cystography
Urinary tract infection is a common and important clinical problem in childhood and a common cause of hospitalizations in children with high fever especially in infancy which they are more prone to suffer from bacteremia [1-3]. We are witnessing a higher incidence of UTI in boys in infancy, in girls the incidence increases with age, uncircumcised children have higher cases of UTI [4,5] in the pediatric population, UTI prevalence is 1% in boys and 4% in girls. The incidence will be higher up to 3%-5% by the age of two years [6,7]. 25% to 30% of children who have had the first infection of pyelonephritis (infection of the kidney tissue) will develop a recurrent infection when the infection occurs during the first year [2,8,9,10]. Up to the age of three months - a significant 3% of all children with high fever suffer from urinary tract infection. Involvement of the renal parenchyma - (pyelonephritis) is accompanied by high fever, chills, Cutis marmorata in infants, vomiting, lethargy, jaundice, and fail to thrive (FTT) [2,3] (Figure 1). Kidney involvement will cause tissue destruction and later may lead to the development of a kidney scar especially in the upper or lower pole of the kidney. The scar can cause high blood pressure and later kidney failure [6,9,11] (Figure 2). Pyelonephritis is attributed to a response to the spread of bacteria to the kidney tissue which will cause increased secretion of cytokines and chemokines by macrophages and neutrophils that have infiltrated the area of inflammation with the formation of micro thrombosis in the capillaries of the renal parenchyma [6,12]. Early diagnosis and quick treatment will prevent the development of a kidney scar. The clinical signs and symptoms of urinary tract infections depend on the age of the child when the most common are high fever, diarrhea, chills, febrile seziure, and Jaundice in infants [6,11,13] (Figure 3). The most common uropathogens in UTI are gram-negative (-) bacteria, the dominant gram-negative – bacteria are E.coli, later Klebsiella pneumonia, Proteus mirabilis, whereas gram-positive (+) bacteria is enterococcus. More resistant bacteria of E.coli or Klebsiella are more visible in recurrent infections.
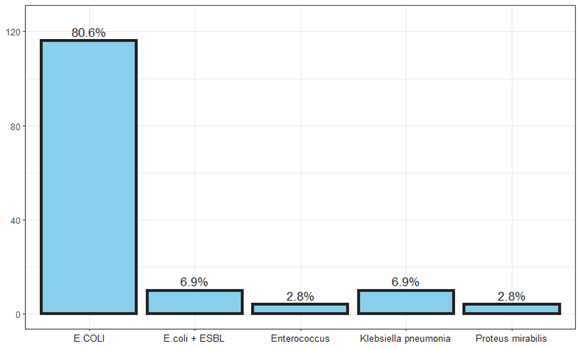
Figure 1: Incidence of urinary tract contaminating bacteria at all ages.
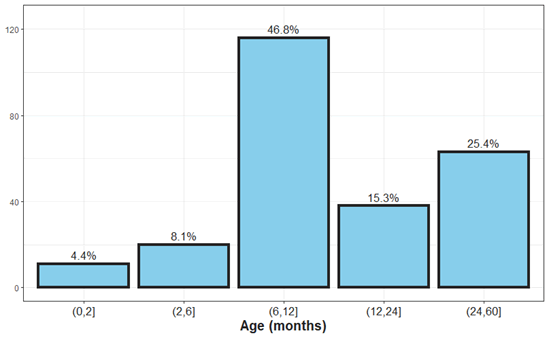
Figure 2: Age distribution of urinary tract infections in children.
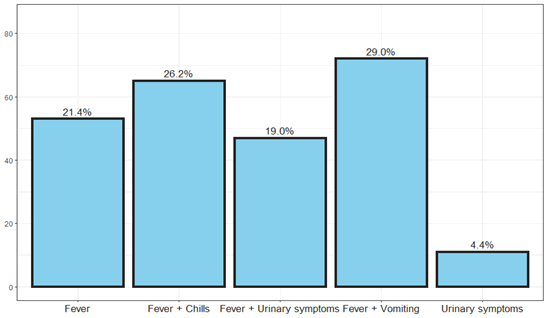
Figure 3: The main symptoms of urinary tract infection in children.
Including indices of inflammation, Cell blood count, ESR, CRP, urinalysis, Urine culture, and blood culture. Kidney and electrolyte functions. Usually, a urine test is performed for both urinalysis and culture, for culture by 3 methods: Urine catheter or mid-current stream test or Suprapubic aspiration of the urinary bladder in infants up to 4 -5 months of age. It's important to avoid performing a urine culture from getting a urine sample from a urine sac [1,2].
A general urine test gives general information about the presence of nitrites or leukocytes, it is considered a not sensitive test that is almost insignificant in infants up to 4 months of age [10,14]. Diagnosis of urinary tract infection is based on urine culture [5,8,13]. Empirical antibiotic therapy is given after taking urine cultures, Blood cultures, Inflammatory and renal function indices. Imaging diagnosis of upper urinary tract infections was made by renal ultrasound, renal mapping, and cystography [1,9,15]. When the renal ultrasound shows the location, size, and involvement of the renal parenchyma, dilation of renal pelvises, and stones [9,16] (Table 1). The second test in renal imaging is renal mapping which is usually done about four to five months after the acute inflammation, the mapping done by DMSA [5,11] (Table 2). The mapping provides information on the renal function of each side and demonstrates cortical filling defects (scars) [2,9]. The third method of imaging is cystography to visualize the presence of vesicoureteral reflux. Today, a new method for detecting reflux by ultrasound was introduced, but it also involves inserting a catheter [5,13]. vesicoureteral reflux (VUR) is considered to cause the development of recurrent urinary tract infections and subsequent development of renal scarring [2,9,16]. The cystography is usually performed 4-6 weeks from the urinary tract infection, the reflux is graded into five degrees while in severe grades IV or V there is the involvement of the collecting system with enlargement and destruction of the renal tissue and renal pelvis, and is characterized by expansion of the ureter and curl. Grade III reflux occurs in the kidney with moderate expansion to the collecting system and slight expansion of the ureter. II degree urine reflux into the ureter and the renal pelvis, without distention, and I degree urine reflux into the ureter only [2,5,17]. The presence of reflux in various and especially high degrees will cause recurrent urinary tract infections, recent studies have found that there is no preference for preventive antibiotic treatment for children with reflux. It has also been noted that the formation of a renal scar is unrelated to the presence or absence of viscerouritral reflux.
| Crosstab | ||||||
|---|---|---|---|---|---|---|
| P=0.459 | US | Total | ||||
| normal | Mild to moderate hydronephrosis | Moderate to severe hydronephrosis | ||||
| Cystography | Normal | Count | 2 | 1 | 0 | 3 |
| % within US | 25.0% | 20.0% | 0.0% | 14.3% | ||
| VUR II-VI | Count | 4 | 4 | 5 | 13 | |
| % within US | 50.0% | 80.0% | 62.5% | 61.9% | ||
| VUR IV-V | Count | 2 | 0 | 3 | 5 | |
| % within US | 25.0% | 0.0% | 37.5% | 23.8% | ||
| Total | Count | 8 | 5 | 8 | 21 | |
| % within US | 100.0% | 100.0% | 100.0% | 100.0% | ||
Table 1: Correlation between ultrasound finding and cystography finding.
| Renal mapping with dmsa and US Crosstabulation | |||||||
|---|---|---|---|---|---|---|---|
| P=0.545 | US | Total | |||||
| normal | Mild to moderate | Moderate to sever | ??????? | ||||
| RENALMapping dmsa | Count | 4 | 1 | 2 | 0 | 7 | |
| % within US | 66.7% | 25.0% | 40.0% | 0.0% | 43.8% | ||
| Pathalogical | Count | 2 | 3 | 3 | 1 | 9 | |
| % within US | 33.3% | 75.0% | 60.0% | 100.0% | 56.3% | ||
| Total | Count | 6 | 4 | 5 | 1 | 16 | |
| % within US | 100.0% | 100.0% | 100.0% | 100.0% | 100.0% | ||
Table 2: Finding that correlate between renal mapping with dmsa and renal us with hydronephrosis . pathological mapping in up to 60%.
The contribution of reflux to the development of a scar or urinary tract infection has not been established [4,9,12]. It is present in the literature that 70% of the renal scars are not related to the finding of visceral reflux and therefore the extent of the contribution of reflux to the scar is questionable [9,11,14] (Table 3).
| Cystography * US Crosstabulation | ||||||
|---|---|---|---|---|---|---|
| P=0.459 | US | Total | ||||
| normal | Mild to moderate hydronephrosis | US moderate to sever hydronephrosis | ||||
| Cystography | normal | Count | 2 | 1 | 0 | 3 |
| % within US | 25.0% | 20.0% | 0.0% | 14.3% | ||
| VUR II-VI | Count | 4 | 4 | 5 | 13 | |
| % within US | 50.0% | 80.0% | 62.5% | 61.9% | ||
| VUR IV-V | Count | 2 | 0 | 3 | 5 | |
| % within US | 25.0% | 0.0% | 37.5% | 23.8% | ||
| Total | Count | 8 | 5 | 8 | 21 | |
| % within US | 100.0% | 100.0% | 100.0% | 100.0% | ||
Table 3: The correlation between renal hydronephrosis in us and vur in cystography.
The imaging protocol in urinary tract infections is as follows: All the children under the age of six months performed the above three tests when an ultrasound was pathological that showed hydronephrosis or the child had no rapid response to antibiotics or an increase in creatinine with an atypical bacterium, Cystography was performed after 4 months [5,6,10] (Figure 4). In children over the age of six months to two years old, an ultrasound is performed, followed by renal mapping, and whether the mapping was pathological (scar, small kidney) or pathological ultrasound (hydronephrosis, ectopic kidney), cystography was performed [6,10,11]. For children with nephronia, abnormal ultrasound findings, fever higher than 39°C within 72 hours of admission, all three tests were done including Ultrasound, mapping, and cystography [5,10,11]. Early detection and initiation of rapid empirical antibiotic therapy to prevent the development of a renal scar [18-25].
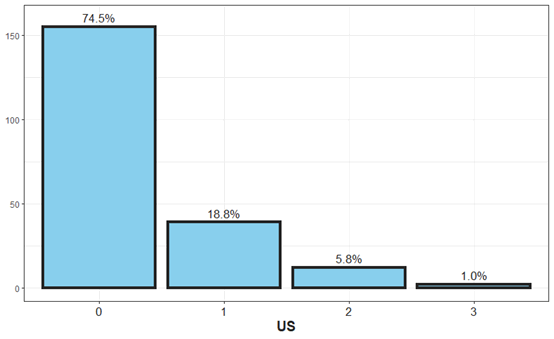
Figure 4: The distribution of hydronephrosis finding in US.
The study is a retrospective in which the cases of children who were hospitalized from infancy to the age of 5 years at pediatric Department, from 2010 to 2019, clinical aspects were examined. All children suffered from symptoms of UTI, children underwent ultrasound imaging, and some underwent renal mapping and cystography. All children underwent urine culture by catheter, SPA, or midstream urine. All children underwent blood tests including a complete blood count with a CRP and ESR, kidney function, and electrolyte. The departmental protocol for initiating antibiotic therapy empirically was the administration of Penibrin (Ampicillin) Garamycin (Gentamicin) until the age of two months, from two months of age treatment with Ceftriaxone, was started and then according to the sensitivity from the laboratory. Preventive treatment is given by chance especially by Resprim. Imaging performed in the hospital most often is a kidney ultrasound. Cystography was done after one month from the infection, According to the indication in the medical literature (atypical infection, severe hydronephrosis, age up to 6 months). Kidney mapping with DMSA after 4-5 months of infection. The children were divided into several groups according to the age. The children whose data were scanned were divided into several age groups:
1-Infancy to 2 months of age 2-age two months to six months 3-age six months to a year 4-age one to two years 5-age two to five years
The duration of hospitalization in each group and the etiology and susceptibility of the bacteria were examined (Figure 5).
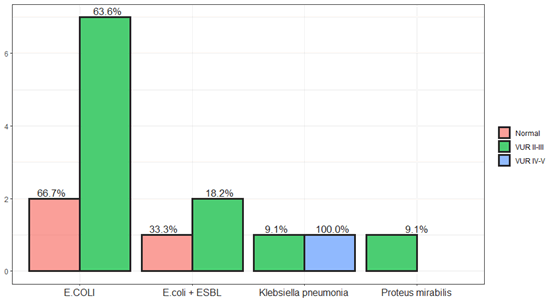
Figure 5: The prevalent frequency of bacteria species in children with vur relux.
The importance of the research
According to the imaging findings of ultrasound and renal mapping combined with laboratory findings, can a cystography examination be saved as a means of diagnosis for urinary tract infection? Statistical analysis and calculation quantitative data will be described using means and standard deviation, median range, qualitative data will be described using frequency and percentages. We will examine differences in quality variables using CHISQUER TEST + FISHER EXACT TEST. Differences vary quantitatively according to the WILCOXON RANK TEST. The relationship between quantitative variables according to the SPERMAN CORRELATION TEST. The study received Helsinki approval (from Ziv Safed Hospital) (Figure 5).
E.coli considered a common uropathogenic bacterium that causes urinary tract infection, Klebsiella pneumoniae is the second most common pollutant in children, Enterococcus and Proteus are equal in their ratio.
Findings and results
In the study, we scanned the files of 248 children up to the age of 5 years, with a higher incidence in girls than boys, 60% were from one year of age and below, and the rest 40% over one to five years (Table 4). The age of the children was divided into groups, from age 0-2 months, from the age of 2-6 months, age of 6 months-1 years of age, and from 1-2 years of age and later up to 5 years of age. The most common reason for admissions was fever, 80% of the children suffered from fever over 39° C and the rest between 38-39° C, another reason for admission was diarrhea in 35%, vomiting in 30%, arrhythmias 18%, refusing to eat 20% convulsions 2%. Re-hospitalizations were in 10% of patients within 3 months of the first infection.
| Renal scan with dmsaand Cystography Crosstabulation | |||||
|---|---|---|---|---|---|
| P=0.629 | Cystography | Total | |||
| VUR II-VI | VUR IV-V | ||||
| Renal mapping | Normal | Count | 2 | 2 | 4 |
| % within Cystography | 50.0% | 66.7% | 57.1% | ||
| pathological | Count | 2 | 1 | 3 | |
| % within Cystography | 50.0% | 33.3% | 42.9% | ||
| Total | Count | 4 | 3 | 7 | |
| % within Cystography | 100.0% | 100.0% | 100.0% | ||
Table 4: Correlation between finding in renal mapping with DMSA and cystography Pathological mapping with 40%reflux presenting.
Microbiological picture
urine culture: 71% catheter, 29% mid-stream urine. The main common uropathogen was E.coli 80%, Klebsiella pneumoniae7%, Enterococcus 3%, Proteus mirabilis3%, ESBL + 7%.
Laboratory
Leukocytosis was present in 70% of patients, The mean leukocytosis was 14,500, Lymphopenia was significant in the presence of reflux. CRP and ESR was taken at 87% were high above 105 mg% ESR above 30, CRP above 4 mg% was considered positive. DIPSTICK was done in the question of leukocyturia or leukocyturia with nitrites. 58% had positive findings of leukocytes and nitrites, in 43% the test was normal. When CRP and ESR were very high in children with high-grade reflux (Table 5).
| Agec | Urine | Total | ||
|---|---|---|---|---|
| leukocyturia | leukocyturia+nitrite | Normal | ||
| (0,2] | 7 63.6 % |
4 36.4 % |
0 0 % |
11 100 % |
| (2,6] | 8 40 % |
12 60 % |
0 0 % |
20 100 % |
| (6,12] | 54 46.6 % |
11 9.5 % |
51 44 % |
116 100 % |
| (12,24] | 18 47.4 % |
0 0 % |
20 52.6 % |
38 100 % |
| (24,60] | 28 45.2 % |
0 0 % |
34 54.8 % |
62 100 % |
| Total | 115 46.6 % |
27 10.9 % |
105 42.5 % |
247 100 % |
| ?2=78.989 · df=8 · Cramer's V=0.400 · Fisher's p=0.000 | ||||
Table 5: Laboratory finding of urine analysis in uti at any age for leukocyte and nitrite.
Renal Imaging methods
Kidney and urinary ultrasound results were performed, 75% of the ultrasound tests in children with urinary tract infection were normal. Mild to moderate dilation was at 20%, moderate to severe including ureter at 5%, and one finding of nephronia. For children who underwent cystography, 30% of the cystography was pathological, 66% suffered from grade II to III, 20% were in grades IV to V. Kidney mapping was done in 20 children, 60% were pathological and 40% were normal (Figure 6).
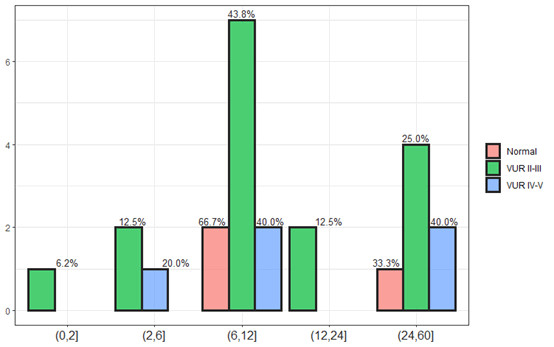
Figure 6: The children age distribution during cystography.
Antibiotic treatment
E.coli was the main contaminant in the first infection and there was high sensitivity to Ampicillin and Gentamicin in addition there was sensitivity to third-generation cephalosporin like Ceftriaxone. Klebsiella pneumonia was the secondary uropthopogen in 30% of cases and was more resistant to Gentamicin, more sensitive to Amikacin almost 98% full sensitivity, enterococcus was 60% resistant to ampicillin and 10% to Amikacin. They were all sensitive to Meropenem. 20 % of E.coli contaminants were resistant to Gentamicin and sensitive to Amikacin and Meropenem (Table 6).
| Agec | Bacteria | Total | ||||
|---|---|---|---|---|---|---|
| E.COLI | E.coli + ESBL | Enterococcus | Klebsiella pneumonia | Proteus mirabilis | ||
| (0,2] | 8 72.7 % |
0 0 % |
1 9.1 % |
0 0 % |
2 18.2 % |
11 100 % |
| (2,6] | 16 80 % |
1 5 % |
1 5 % |
2 10 % |
0 0 % |
20 100 % |
| (6,12] | 52 82.5 % |
4 6.3 % |
1 1.6 % |
6 9.5 % |
0 0 % |
63 100 % |
| (12,24] | 17 73.9 % |
4 17.4 % |
0 0 % |
2 8.7 % |
0 0 % |
23 100 % |
| (24,60] | 23 85.2 % |
1 3.7 % |
1 3.7 % |
0 0 % |
2 7.4 % |
27 100 % |
| Total | 116 80.6 % |
10 6.9 % |
4 2.8 % |
10 6.9 % |
4 2.8 % |
144 100 % |
| ?2=26.256 · df=16 · Cramer's V=0.214 · Fisher's p=0.069 | ||||||
Table 6: The contaminating bacteria at any age are ecoli and klebseila pneumonia.
Our study is a retrospective study of 248 children who were hospitalized in the pediatric department of Ziv Medical Center in northern Israel from 2010 to 2019. Early treatment of urinary tract infection will prevent the development of a kidney scar. 60% of the hospitalizations were under one year of age, 25% of the hospitalizations were from two to five years of age and 15% between the ages of one and two years. In terms of symptoms and signs, 90% of the children had fever at all ages, 26% had fever and chills, the main uropathogen was E.coli followed by Klebsiella pneumonia and Enterococci, Proteus mirabilis while Enterococci tends to be in boys (Figure 7).
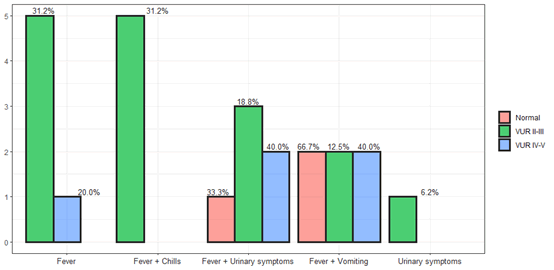
Figure 7: Percent complains during anamnesis.
The E.coli and the Klebsiella were 25% resistant especially to the Ceftriaxone most of them ESBL+.
The bacterial resistance of E.coli and Klebsiella was more for Ampicillin and Gentamicin, Augmentin and Ceftriaxone, less for Meropenem and Amikacin. 20% there are episodes of recurrent urinary tract infections after the first infection that the generators are Klebsiella and E.coli ESBL+. Renal reflux is a common urinary anomaly that is a risk factor for urinary tract infection. Renal hydronephrosis with ureter dilation predicts the presence of reflux. Cystography Imaging method for cell reflux. In terms of imaging, ultrasound mapping and cystography are necessary under the age of six months, and further to laboratory clinical correlation. There is a greater tendency for reflux in girls compared to boys (Figure 8). The recurrence of urinary tract infection was 20%-25% after the first hospitalization and especially for 6 months after the first infection. leukocytosis with high CRP lymphopenia, and ESR supported the presence of high-grade reflux. Prophylactic treatment with antibiotics did not reduce the recurrence of the urinary tract infection but caused an increased risk of bacterial resistance. Children in the first year with reflux who received prophylactic antibiotics 48% developed a recurrent infection after 3-6 months of the first urinary tract infection. The common contaminants in cases of recurrence of urinary tract infection were E.coli and Klebsiella of the resistant strains type. DMSA was pathological in 70% in the presence of high-grade reflux while in children without reflux pathological mapping was 30%. In children up to the age of two years, a strong correlation was found between reflux and pathological mapping, so pathological mapping can predict the presence of reflux in high percentages (Figure 9).
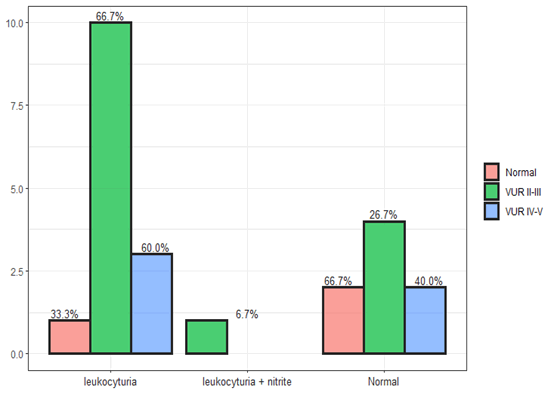
Figure 8: laboratory finding in urine test with coloration to VUR.
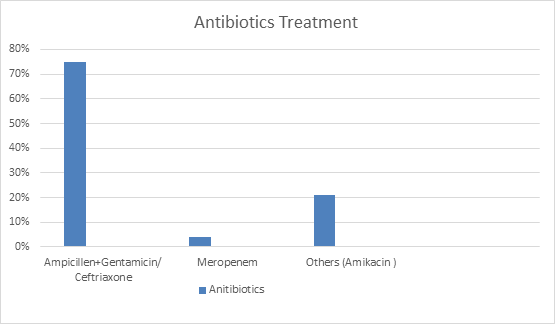
Figure 9: The standard treatment was ampicillin and gentamycin in UTI recurrent infection of UTI amikacin was more sensitive.
Primary urinary tract infection at all ages is caused by E.coli, which is sensitive to empirical antibiotics, Including Ampicillin and Garamycin. In recurrent infections the uropathogens are more diverse and tend to be more resistant. The tendency today to start Amikacin instead of Gentamicin especially in infants under 6 months of age. We can see Resistance to antibiotic treatment in recurrent urinary tract infection.
Hight level of ESR and CRP, Lymphopenia with leukocytosis predict reflux with a picture of pyelonephritis. No cystography should be performed on any infection in question of VUR, renal mapping with DMSA is the dominant test in addition to ultrasound in urinary tract infection. Ultrasound findings of hydronephrosis do not always predict the presence of VUR reflux. The combination of a high ESR and pathological mapping and ultrasound findings will give the reinforcement to perform cystography on the question of reflux. Early diagnosis and appropriate treatment will prevent the development of a kidney scar.
Published: 19-Jul-2021 , DOI: 10.35248/2161-0665.21.11.391
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.