
Journal of Clinical Toxicology
Open Access
ISSN: 2161-0495

ISSN: 2161-0495
Case Report - (2022)Volume 12, Issue 2
Calcium Channel Blocker overdose is one of the leading causes of death among cardiovascular medications. However, there is still a paucity of information regarding management of calcium channel blocker overdoses. Furthermore, management of combination antihypertensive medication overdoses has not been well-documented. We report a case of a twenty-five-year-old woman who was admitted to the emergency department due to a large overdose of a combination medication, consuming 3,600 mg olmesartan, 900 mg amlodipine, and 2,250 mg hydrochlorothiazide. The patient required intubation and aggressive vasopressor support with adjunctive insulin, dextrose, glucagon, activated charcoal, and calcium chloride infusions. After a 12.9 day admission, the patient fully recovered and was discharged.
Amlodipine; Olmesartan; Hydrochlorothiazide; Overdose; Toxicology
Calcium Channel Blocker (CCB) overdose is one of the leading causes of overdose death among cardiovascular medications. Though the class constitutes only 13.8% of cardiovascular drug overdoses either alone or in combination, CCB overdoses account for 42.2% of deaths in these patients [1]. Amlodipine is a commonly prescribed Dihydropyridine (DHP) CCB used to treat hypertension, angina pectoris, cardiac arrhythmias, and other cardiovascular disorders. However, unlike the other DHP CCB’s, amlodipine has a unique pharmacokinetic profile characterized by a slower onset of action (peak concentrations between 6 to 9 hours), a large volume of distribution (21 liters/ kg), and an extensive half-life (35-45 hours). Amlodipine demonstrates linear pharmacokinetics and has a strong correlation between the oral dose given and the plasma concentration-time curve [2]. The clinical picture of previously documented CCB intoxication cases consist of severe hypotension, arrhythmias, pulmonary congestion, renal failure, liver impairment, and metabolic acidosis [3,4]. Limited large overdose cases of amlodipine have been reported in the literature.
A 25-year-old, Hispanic woman with no significant medical history took approximately 90 tablets of her grandmother’s antihypertensive medication Tribenzor™, a combination antihypertensive product that contains olmesartan, amlodipine, and hydrochlorothiazide (40/10/12.5 mg). The patient presented to the Emergency Room (ER) 2 hours after ingestion. On first evaluation, the patient was alert with a Blood Pressure (BP) of 100/50, Heart Rate (HR) 113, Respiratory Rate (RR) 18, oxygen saturation (O2 Sat) 96% on 4 liters of oxygen. The patient’s BP progressively declined and she was intubated for airway protection and started on assist control mode for ventilation. The ER course of treatment consisted of gastric lavage, 2 grams of intravenous calcium chloride, 50 g of activated charcoal through orogastric tube, 4 liters of normal saline boluses, norepinephrine infusion (15 mcg/kg/min titrated up to 150 mcg/kg/min while in the ER), and an insulin drip (8 units/hr, titrated up to 30-40 units/hr). A dextrose infusion was initiated to maintain euglycemia. Normal saline boluses and a norepinephrine infusion were both started for blood pressure support. After two hours, she was transferred to the Medical Intensive Care Unit (MICU) for further management. Her labs revealed a noncontributory complete blood count, anion gap metabolic acidosis, with a glucose level of 160 mg/dl and calcium concentration of 10 mg/dl. Acetaminophen, salicylate, and alcohol levels were negative.
The norepinephrine infusion was titrated upto 200 mcg/min. She was started on a dopamine drip titrated up to a significantly high dose of 50 mcg/kg/min. Phenylephrine and vasopressin were also added and titrated up to 200 mcg/ min and 0.06 units/min, respectively. The aggressive vasopressor support continued for several days (Figure 1). Additionally, the patient experienced significant hypoxia due to pulmonary edema 13 hours after admission. She required high positive end-expiratory pressure on assist/control ventilation and was started on high frequency oscillator mode. Despite being on high frequency oscillator mode, her SpO2 was around 78%. The patient was oliguric with a central venous pressure of 19 mmHg and subsequently initiated on diuretics, initially with bumetanide and later with furosemide.
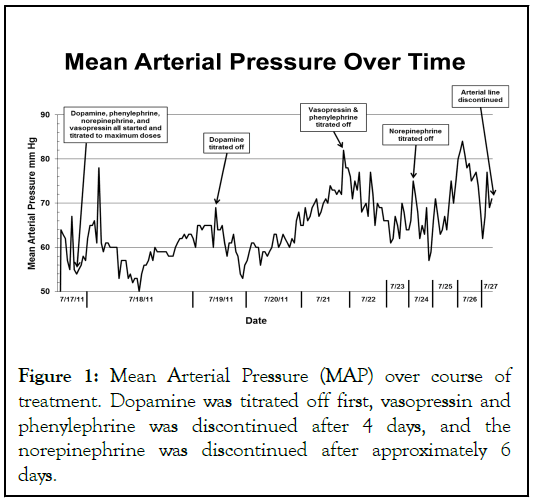
Figure 1: Mean Arterial Pressure (MAP) over course of treatment. Dopamine was titrated off first, vasopressin and phenylephrine was discontinued after 4 days, and the norepinephrine was discontinued after approximately 6 days.
Adjunctively, the patient received a calcium chloride infusion with hourly serum calcium monitoring, glucagon, and insulin with dextrose infusions. Intravenous calcium was given to maintain a calcium level goal above 11 mg/dL. The patient became more responsive and on day three, the patient had improved oxygenation, increased urine output, and required less vasopressors. By day six, vasopressors were slowly weaned off and the patient was extubated. The patient remained in the ICU for 11 days and then was transferred to the floor. The patient had a full recovery. Her total hospital admission was a duration of 12.9 days.
Management of patients with severe CCB toxicity is challenging. Patients who have ingested toxic doses of CCBs commonly manifest symptoms of bradycardia, conduction delay, peripheral vasodilation, hypoinsulinemia and hyperglycemia [4]. Patients with mild to moderate CCB toxicity may experience asymptomatic bradycardia or mild hypotension. Severe toxicity can have a profound effect on heart rate and can cause dysrhythmias. Metabolic acidosis can be common as well, secondarily to poor perfusion. Combination product overdoses may present differently than a CCB or Angiotensin-Converting Enzyme Inhibitor (ACE-I) overdose alone. In the case of combination products containing both CCB and ACE-I, the ACE-I component may confound the traditional response to antidotal and supportive therapy recommended for CCB overdoses [5]. Gastric lavage and activated charcoal administration are both methods that have been previously documented for use in standard overdose treatments. Since amlodipine is highly protein bound (93% bound to plasma protein), hemodialysis is not a treatment option [6].
Administration of activated charcoal and gastric lavage should be considered for all cases of CCB overdose and is most effective when given within 1-2 hours after ingestion [7]. Regardless of whether a patient is experiencing symptoms, an aggressive approach to care for CCB overdose should be taken, depending on patient-specific factors such as age, comorbidities, coingestion of other cardiovascular medications, and the magnitude of toxic ingestion. Prompt treatment is crucial as the clinical presentation may initially be asymptomatic with delayed presentation of cardiovascular collapse. Manifestations of toxicity may be delayed up to 16 hours after the ingestion with sustained-release formulations [8]. In this case, the patient ingested a combination pill containing a CCB, ACE-inhibitor, and diuretic which may have resulted in the unusual requirement of multiple, high-dose vasopressor support.
Previous case reports regarding amlodipine overdose with coingestion of cardiovascular medications have reported treatment with fluid resuscitation, vasopressor support, diuretics, calcium gluconate, glucagon, and lipid emulsion bolus [9-11]. More recently, there is increasing use of lipid emulsions for CCB overdose patients. Previous case reports have shown benefit of use, including reduction in vasopressor requirements; however, there is certainly still a paucity of information regarding the mechanism of action and risks of use [11-13].
This patient exhibited decreased mentation and was hemodynamically unstable requiring intubation for airway protection. Refractory hypotension was initially treated with fluid boluses, and then treated with intravenous calcium, highdose insulin infusions, dextrose, glucagon and vasopressors. Administration of intravenous calcium is a standard treatment option but its clinical efficacy has not proven to be consistent and specific dosing recommendations are not known [1,10]. Close monitoring of the serum or ionized calcium concentration every two hours and serial ECGs are necessary to avoid clinically significant hypercalcemia.
Calcium Channel Blockers are being increasingly used for treatment of hypertension and are often combined with other antihypertensive medications in a combination pill. CCB overdose may cause severe hypotension along with bradycardia, pulmonary edema, renal failure and other signs of heart failure.
Based on this case report and review of available literature, patients with near fatal CCB ingestion and co-ingestion of additional cardiovascular medications generally have positive clinical outcomes with appropriate initiation of high-dose vasopressors, calcium, and high-dose insulin and dextrose infusions.
Citation: Zhong C, Seifert CF (2022) Large Overdose of a Combination Antihypertensive Medication. J Clin Toxicol. 12:505.
Received: 15-Mar-2022, Manuscript No. JCT-22-16417; Editor assigned: 17-Mar-2022, Pre QC No. JCT-22-16417; Reviewed: 31-Mar-2022, QC No. JCT-22-16417; Revised: 07-Apr-2022, Manuscript No. JCT-22-16417; Published: 14-Apr-2022 , DOI: DOI: 10.35248/2161-0495.22.12.505.
Copyright: © 2022 Zhong C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.