Journal of Nutrition & Food Sciences
Open Access
ISSN: 2155-9600
ISSN: 2155-9600
Research Article - (2020)Volume 10, Issue 5
Background: Nutraceuticals containing hydroxytyrosol have been shown to exert a wide range of beneficial effects on the cardiovascular system like lipid modification and cholesterol reduction. The aim of this randomized doubleblind, placebo-controlled parallel study was to investigate the effects of a pure, synthetic form of hydroxytyrosol (HTEssence®) on the lipid profile in subjects with elevated LDL-cholesterol level.
Methods: 92 subjects underwent a 12-week intervention with hydroxytyrosol or placebo. At baseline, after 6 and 12 weeks of intervention the lipid profile of the subjects was assessed.The study was conducted in accordance with the guidelines for Good Clinical Practice (GCP) andwas registered in the German Clinical Trials Register (DRKS00015251).
Results: A significant delta change of LDL-cholesterol levels was observed in the hydroxytyrosol group after 12 weeks of intervention comparedto the placebo group (p=0.0454). The absolute difference between placebo and hydroxytyrosol accounted for 8.46mg/dL.With regard to the changes within the intervention groups after 12 weeks, a slight decrease of LDL-cholesterol could be observed in the hydroxytyrosol group (-2.70 mg/dL, 95% CI: -8.15- 2.75) and, in contrast, an increase of this parameter in the placebo group (5.76 mg/dL, 95% CI: 0.23– 11.28). Regarding the other parameters of lipid status including total cholesterol, HDL-cholesterol, LDL/HDL-ratio and triglycerides, no lipid modifying effects were observed.
Conclusion: Data of the current clinical study support the evidence for hydroxytyrosol being a nutraceutical with anti-atherogenic effects.
Hydroxytyrosol; LDL cholesterol;HDL cholesterol; Lipid status; Blood pressure
Elevated cholesterol levels, in particular high levels of low-density lipoprotein (LDL)-cholesterol, is one of the major risk factors for atherosclerosis and related cardiovascular diseases, such as coronary heart disease or cerebrovascular disease. It has been shown that a reduction in LDL-cholesterol is effective in decreasing the risk of death from coronary heart disease [1]. Dietary adaption as a part of a general lifestyle modification is often a successful basic step in cholesterol lowering therapy. Nevertheless, the prescription of statins is still first-line treatment, albeit this therapy is accompanied by partly strong side effects and poor tolerability [2]. Clinically proven food ingredients and nutraceuticals like polyphenols were shown to effectively and safely support the management of elevated LDL-cholesterol [3]. Moreover, epidemiologic studies have shown a lower incidence of cardiovascular diseases in individuals consuming a Mediterranean diet [4,5]. It has been suggested that these effects are partly attributable to the olive oil component of the diet [6]. Hydroxytyrosol is one of the main phenolic components of olive oil and is present in olive fruits and leaves, as well as in red wine.
The lipid-modifying effects of hydroxytyrosol from the olive plant, such as reduction of LDL-cholesterol, inhibition of LDL peroxidation, reduction of lipid accumulation and increase of high-density lipoprotein (HDL)-cholesterol were reported in previous animal and in vitro studies [7-9]. LDL-cholesterol-lowering effects have been confirmed in healthy elderlyconsuming extra virgin olive oil [10], and in borderline or stage-1 hypertensive subjects consuming olive leaf extract [11,12]. In addition, in prehypertensive males, the intake of a phenolic-rich olive leaf extract reduced cardiovascular risk factors, such as blood pressure and plasma levels of total cholesterol, LDL-cholesterol and triglycerides [13]. Other studies also investigated the effect of hydroxytyrosol in combinations with other nutraceuticals like phytosterols or vitamins. A recent study among patients with primary hypercholesterolemia at low-to-moderate cardiovascular risk receiving a nutraceutical combination containing phytosterols, monacolin K from red yeast rise, vitamin E and hydroxytyrosol over the course of three months, demonstrated a reduction of total cholesterol and LDLcholesterol by 11.4% and 14.1% [14]. Another study reported the reduction of total cholesterol by 20.5%, triglycerides by 13.1% and LDL-cholesterol by 17.7% after a 12-week supplementation with a nutraceutical combination containing vitamins and polyphenols, one of which was hydroxytyrosol [15]. However, despite these evidences, current literature of the lipid-modifying effects of olive polyphenols is somewhat controversial, as a number of studies have failed to show any hydroxytyrosol-mediated effects on lipid status. An in vivo study published by Lopez-Huertas et al. using 45 mg of purified hydroxytyrosol (99.5%), showed no effect on blood lipids after 8 weeks of supplementation [16]. Another study including 12 hypercholesterolaemic subjects, who received three olive oil formulations differing in the concentration of phenolic compounds for a 3 week-period each, followed by 2-week washout periods, did not show any statistical changes regarding the lipid status parameters [17]. Still, oxidized LDL decreased after the intervention with virgin olive oil containing a 1:1 mixture of phenolic compounds from olive oil and thyme [17]. A cross-over study published by Vázquez-Vela with 22 subjects, who consumed either hydroxytyrosol-enriched (45–50 mg/d of hydroxytyrosol) or non-enriched sunfloweroil over the course of 3 weeks, did not observe any changes in serum cholesterol and lipoprotein concentrations [18]. The inconsistent data on the hydroxytyrosol effect may be due to the variability of formulations and delivery vehicles used in previous studies, as most studies were performed either with olive oils or with olive leaf extract. However, olives do not only contain free hydroxytyrosol but also other phenolic compounds like oleuropein and ligstroside, which are enzymatically converted to theirunglycosylated forms (oleuropein aglycone)and finally into hydroxytyrosol during maturation or processing of the fruit. The degree of olive processing (like oil ripening) may therefore strongly affect the content of different polyphenols [19]. Olive leaves also contain such bioactive molecules like oleuropein, ligstroside and free hydroxytyrosol, and their amounts vary depending on the origin, on climatic and storage conditions, as well as on the degree of processing [19]. Furthermore, some of the hydroxytyrosol formulations described in literature contain additional bioactive compounds like vitamins or other polyphenols, which may also account for the beneficial effects of the investigated products. Therefore, more studies are needed, which, in an isolated manner, investigate the impact of single phenolic compounds of olive oil to provide product specific data, which may help to better understand the mechanism of lipid modifying properties of hydroxytyrosolcontaining nutraceuticals.
Aim of the current study
As the isolation of pure hydroxytyrosol from olive plants is usually costly and often provides only low amounts of the pure substance, there is a growing interest for other sources of hydroxytyrosol. Several methods for the synthesis of hydroxytyrosol are described in the literature [20-23], some of which are promoted as cheap and convenient [20,23]. HTEssence® is a highly pure nature-identical synthetic form of hydroxytyrosol. In a previous pilotclinical study performed in 2016, we demonstrated the LDL-cholesterol lowering effect of a daily intake of 30 mg of this product after 4 weeks of intervention in individuals with normal cholesterol and lipid status [24]. The current study aimed to confirm lipid modifying effects of synthetic hydroxytyrosol HTEssence®, in a target population at low to moderate risk for cardiovascular diseases aiming to control their elevated LDL-cholesterol levels by lifestyle changes.
Study design
The study was performed as a randomized, double-blind, placebocontrolled study in parallel design at the study site of BioTeSys GmbH (Esslingen, Germany). The study was conducted in accordance with the guidelines for Good Clinical Practice (GCP) set forth by the International Conference on Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), and the Declaration of Helsinki regarding the treatment of human subjects in a study. The trial was reviewed by the Institutional Review board (IRB) of the Landesärztekammer Baden-Württemberg without concerns. The study was registered in the German Clinical Trials Register (DRKS00015251). During the intervention period of 12 weeks study participants received hydroxytyrosol or placebo daily. At baseline, after 6 weeks (visit 2) and after 12 weeks (visit 3) of supplementation different outcome measures were evaluated. Baseline data of fat status(total cholesterol, LDL-cholesterol, HDL-cholesterol and triglycerides) were collected twice (screening visit and visit 1 prior to the intervention start) to account for physiologic variability and allow for reliable baseline measurements. For standardization, subjects were advised to eat a standardized evening meal at least 10 h before each sampling time. The meal was controlled for fat content. Subjects were also asked to keep their nutrition and exercise habits unchanged during the study participation.
Subjects
From September 2018 to January 2019, 126 subjects were screened for eligibility at BioTeSys GmbH (Esslingen, Germany), wherefrom 92 subjects were randomized (Figure 1). Informed consent from each subject participating in the study was obtained after written and oral explanation of the aims, methods, benefits and potential hazards of the study. Healthy men and womenat the age of 18-75 with a BMI between 18.5 and 35 kg/m2 who showed mildly elevated LDL-cholesterol levels (LDL-cholesterol levels ≥115 mg/dL and <190 mg/dL) were eligible to participate. Further, the study population was selected based on the ESC (European Society of Cardiology) guidelines for the management of dyslipidaemia. Accordingly, only individuals at low to moderate risk (Systematic Coronary Risk Evaluation (SCORE) Germany ≥1% and <5%) were included in the study. The main exclusion criteria for study participation were fasting triglyceride levels >350 mg/dL, intake of lipid-lowering drugs or dietary supplements, extensive smokingbehaviour (>10 cigarettes/day), recent body weight change >4.5 kg, and a relevant history or presence of any medical disorder potentially interfering with this study, like heavy depression, diabetes, active cancer or heavy cardiovascular diseases (e.g. stroke, heart attack).
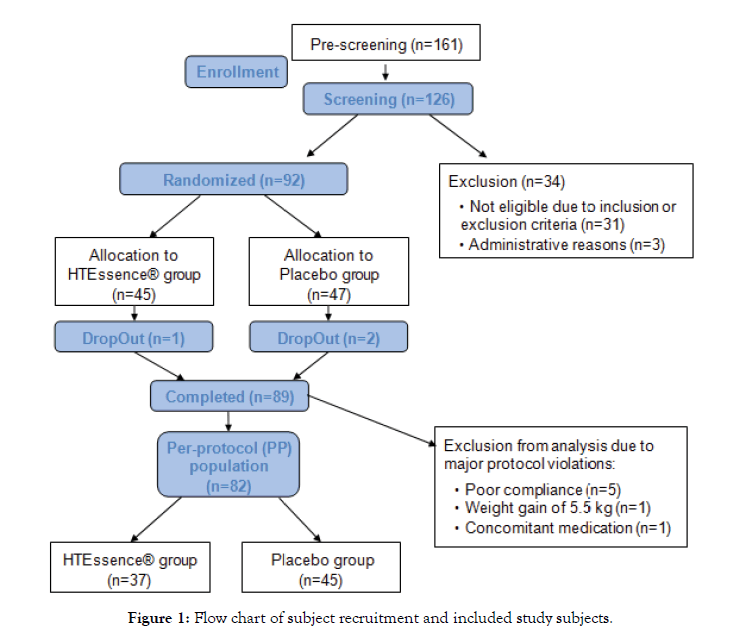
Figure 1: Flow chart of subject recruitment and included study subjects.
Investigational product
The investigational product (30 mg/day hydroxytyrosol; HTEssence®, Wacker Chemie AG, Munich, Germany)was a spray-dried powder with 20% synthetic hydroxytyrosol content on maltodextrin for use in dietary supplements. Maltodextrin with no active substances (355 mg/day) was used asplacebo preparations. All products were provided in vegetable hydroxypropyl methylcellulose(HPMC) capsules with the same shape and appearance to allow double blind performance. Study preparations were taken up by means of 2 capsules twice daily, in the morning and in the evening, together with a meal. Manufacturing of the study products was carried out in compliance with Good Manufacturing Practice (GMP) conditions and all ingredients and capsules were of food grade quality and met European food regulations.
To allow for standardization at visit 2 and 3, only in the evening prior to visit 2 and 3, subjects were instructed to take the capsules 12 h prior to blood sampling. In the morning of visit 2, the products were taken after the blood sampling.
Sample and data collection
Venous blood samples were collected at screening and at the study visits after an overnight fast of at least 10 h in S-Monovette® serum tubes from Sarstedt (7.5 mL Z-Gel for serum). After clotting for 30 min at room temperature, serum was centrifuged for 10 min at 3000 × g and 4°C. Blood routine parameters and the lipid status parameters LDL-cholesterol, total cholesterol, HDL-cholesterol, and triglycerides were analyzed at an accredited laboratory (Synlab Medizinisches Versorgungszentrum Leinfelden, Germany) using ADVIA2400 clinical chemistry analysis system from Siemens.LDLcholesterol was calculated according to Friedewald calculation. Additionally, the effect on the blood pressure was evaluated. Vital signs were determined at each visit in sitting position after at least 5 min rest under fasting conditions.
Methods for safety (Adverse events, concomitant medication and tolerability)
During the study intervention, the subjects documented any adverse events (AEs) and concomitant medication in diaries. At study visits, these entries were monitored and judged by the authorized study staff. Tolerability of the study products was assessed at the end of the intervention period.
Sample size calculation and randomization
Sample size calculation was based on the results of the pilot study and effects described in literature. Assuming an effect size of 0.64 a sample size of 40 per group would provide approximately 80% power to detect a difference between hydroxytyrosol and placebo by using a significance level of 5%. Considering possible drop outs, a total sample size of 92 subjects was calculated.When subjects fulfilled all inclusion and none of the exclusion criteria, being eligible for the study they were allocated randomly to one of the two study groups.The randomization was stratified by gender (male or female) and LDL-cholesterol levels (LDL-cholesterol ≤ 160 mg/ dL or>160 mg/dL at screening). The randomization scheme was created by the software DatInfRand list Version1.2 (DatInf GmbH, Tübingen, Germany).
Statistical analysis
The primary end point was defined as the change of LDL-cholesterol from baseline to end of intervention (visit 3 after 12 weeks of supplementation). To test if there was a difference in the primary endpoint between hydroxytyrosol and placebo, analysis of covariance (ANCOVA) was applied with the baseline LDL-cholesterol level as covariate. For the analysis the baseline corrected values were used, whereas baseline was calculated as mean value between screening and visit 1 (prior to the start of intervention). Secondary variables were total cholesterol, HDL-cholesterol, triglycerides and vital signs (systolic and diastolic blood pressure). For evaluation of changes between groups, the same statistical analysis as described for the primary endpoint was applied. 82 subjects were evaluated in the per-protocol-population (PP population). The main reasons for the exclusion from the analysis are summarized in Figure 1. Additionally, two subjects were excluded from the analysis of LDLcholesterol due high intra-individual variability of LDL-cholesterol values at baselineand one subject was excluded from the analysis of blood pressure; due to start of intake of hypertensive medication during study conduct. All statistical tests were performed two-sided. A significance level of 0.05 was used. The analysis was performed with IBM SPSS statistics 24 statistical software (Armonk, NY, USA) and Graph Pad Prism software (La Jolla, CA, USA).
Subject characteristics
From 92 randomized subjects, 89 subjects completed the study in its entirety (Figure 1). Three subjects dropped out, one subject from the hydroxytyrosol group due to poor compliance to the study procedures and two subjects from the placebo group because the product was poorly tolerated. These two subjects complained about gastrointestinal findings like stomach ache and diarrhoea. It is highly unlikely that these findings are attributable to the placebo product (maltodextrin). Rather, it is more likely to be due to other coincidental concomitant reasons. However, due to the participation in the study, the gastrointestinal complaints were projected onto the study product and the subjects therefore discontinued the study.Baseline characteristics of the PPpopulation are presented in Table 1. There were 14 men and 23 women in the hydroxytyrosol group and 18 men and 27 women in the placebo group. From baseline characteristics study groups were comparable with no significant differences between the study groups. The Systemic COronary Risk Estimation (SCORE) was used for calculation of the 10-year risk of fatal CVD with reference to age, gender, smoking habits, blood pressure and cholesterol levels. Table 1 also summarizes the distribution of CV risk SCORE among the study population. Overall, the compliance of intake of study products was good with an average compliance of 97.3% (95% CI: 96.1-98.5) in the placebo and 98.6% (95% CI: 97.5-99.7) in the hydroxytyrosol group. Only 5 subjects showed compliance below 80% and were therefore excluded from the analysis population.
| Variables | Placebo (n=45) | Hydroxytyrosol (n=37) | ||
|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | |
| Age [years] | 55.4 | 51.7 to 59.2 | 52.8 | 48.2 to 57.3 |
| BMI [kg/m2] | 24.8 | 23.8 to 25.8 | 25.2 | 24.1 to 26.3 |
| Compliance [%] | 97.3 | 96.1 to 98.5 | 98.6 | 97.5 to 99.7 |
| Hemoglobin [g/dL] | 14 | 13.7 to 14.3 | 14.1 | 13.8 to 14.5 |
| AST [U/L] | 22.4 | 20.6 to 24.1 | 23.3 | 20.8 to 25.8 |
| ALT [U/L] | 24.1 | 21.1 to 27.0 | 27.6 | 23.5 to 31.7 |
| CV Risk score [n] | ||||
| 0% | 18 | 16 | ||
| 1% | 10 | 12 | ||
| 2% | 5 | 4 | ||
| 3% | 5 | 3 | ||
| 4% | 7 | 2 | ||
Table 1: Demographic data and baseline characteristics.
Lipid modifying properties of hydroxytyrosol after a 12-week intervention with hyper cholesterolaemic subjects
Table 2 shows an overview of lipid status parameter during the intervention phase. Overall, a slight decrease of LDL-cholesterol was observed over time by trend (p=0.0991) in the hydroxytyrosol group and a slight continuous increase of LDL-cholesterol in the placebo group by trend (p=0.0905). Comparison of changes in LDL-cholesterol levels between groups showeda significant delta change of LDL-cholesterol levels after 6 weeks (p=0.0139) and after 12 weeks (p=0.0454) (Figure 2). In contrast, the comparison of delta changes of total cholesterol levels between both study groups did not show any differences neither after 6 weeks (p=0.2817) nor after 12 weeks (p=0.7050) of supplementation.Further, consistently with the previously observed effects of the investigational product in healthy subjects [24], no significant inter-group differences in HDL-cholesterol levels were observed in the current study. There were also no significant effects of hydroxytyrosol on LDL/HDLratio compared to the placebo group. Finally, no changes were observed regarding triglyceride levels or blood pressure between hydroxytyrosol and placebo (Table 2).
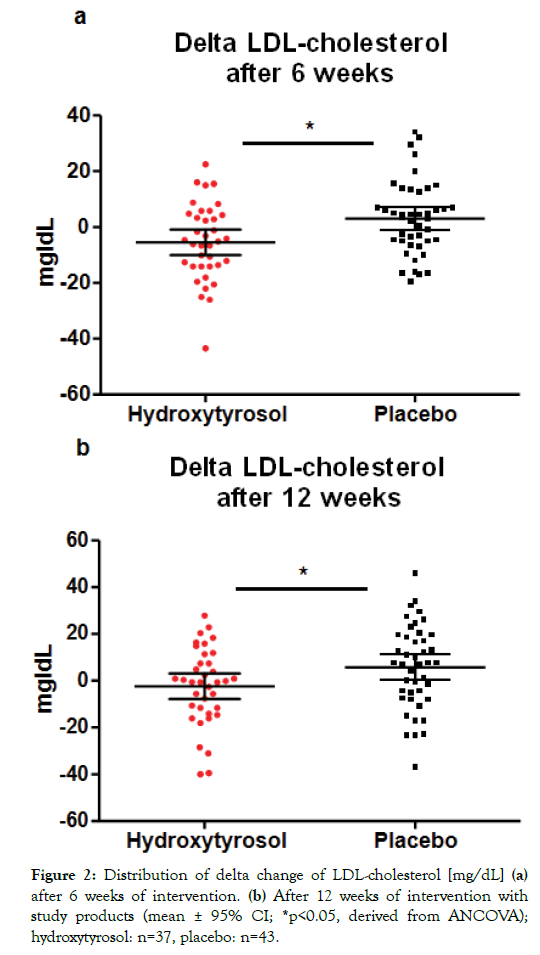
Figure 2: Distribution of delta change of LDL-cholesterol [mg/dL] (a) after 6 weeks of intervention. (b) After 12 weeks of intervention with study products (mean ± 95% CI; *p<0.05, derived from ANCOVA); hydroxytyrosol: n=37, placebo: n=43.
| Lipid status parameters | Baseline | 6 weeks | 12 weeks | Delta change after 6 weeks | p-Value between groups1 | Delta change after 12 weeks | p-Value between groups1 |
|---|---|---|---|---|---|---|---|
| Total cholesterol [mg/dL] | |||||||
| Hydroxytyrosol (n=37) | 219.3 (210.2 to 228.4) | 215.3 (206.0 to 224.5) | 224.5 (214.9 to 234.0) | -4.04 (-10.37 to 2.29) | 0.2817 | 5.15 (-2.35 to 12.64) | 0.705 |
| Placebo (n=45) | 221.7 (214.8 to 228.7) | 221.2 (213.2 to 229.1) | 228.2 (219.2 to 237.3) | -0.53 (-4.46 to 3.39) | 6.51 (0.54 to 12.49) | ||
| LDL-cholesterol [mg/dL] | |||||||
| Hydroxytyrosol (n=37) | 150.3 (143.6 to 156.9) | 145.0 (138.7 to 151.3) | 147.6 (140.7 to 154.4) | -5.30 (-9.87 to -0.73) | 0.0139 | -2.70 (-8.15 to 2.75) | 0.0454 |
| Placebo (n=43) | 145.3 (140.0 to 150.7) | 148.4 (142.0 to 154.8) | 151.1 (143.0 to 159.2) | 3.06 (-0.99 to 7.11) | 5.76 (0.23 to 11.28) | ||
| HDL-cholesterol [mg/dL] | |||||||
| Hydroxytyrosol (n=37) | 63.8 (58.6 to 68.9) | 62.4 (56.4 to 68.5) | 62.7 (57.2 to 68.2) | -1.32 (-3.53 to 0.88) | 0.6054 | -1.03 (-3.46 to 1.41) | 0.3948 |
| Placebo (n=45) | 66.2 (60.8 to 71.5) | 65.6 (60.2 to 71.0) | 66.2 (61.0 to 71.3) | -0.59 (-2.33 to 1.15) | -0.01 (-1.53 to 1.51) | ||
| Triglycerides [mg/dL] | |||||||
| Hydroxytyrosol (n=37) | 102.9 (92.2 to 113.6) | 107.1 (95.1 to 119.0) | 111.4 (99.3 to 123.6) | 4.16 (-5.61 to 13.94) | 0.8205 | 8.54 (-0.36 to 17.45) | 0.282 |
| Placebo (n=45) | 112.1 (99.3 to 124.9) | 111.1 (99.5 to 122.7) | 112.1 (99.9 to 124.3) | -1.06 (-11.94 to 9.83) | -0.03 (-8.49 to 8.43) | ||
| LDL/HDL-ratio | |||||||
| Hydroxytyrosol (n=37) | 2.50 (2.27 to 2.72) | 2.50 (2.26 to 2.75) | 2.51 (2.27 to 2.74) | 0.007 (-0.070 to 0.084) | 0.1908 | 0.010 (-0.098 to 0.119) | 0.2521 |
| Placebo (n=43) | 2.32 (2.11 to 2.53) | 2.41 (2.18 to 2.64) | 2.41 (2.17 to 2.66) | 0.090 (-0.010 to 0.189) | 0.089 (-0.005 to 0.183) | ||
| Blood pressureSys [mmHg] | |||||||
| Hydroxytyrosol (n=37) | 122.2 (118.4 to 126.1) | 120.9 (116.6.to 125.2) | 123.4 (118.6 to 128.1) | -1.38 (-3.72 to 0.96) | 0.4985 | 1.14 (-2.49 to 4.76) | 0.4328 |
| Placebo (n=44) | 125.1 (120.2 to 129.9) | 125.0 (119.2 to 130.9) | 127.5 (122.6 to 132.3) | -0.03 (-3.06 to 2.99) | 2.40 (-0.45 to 5.25) | ||
| Blood pressure Dia [mmHg] | |||||||
| Hydroxytyrosol (n=37) | 77.8 (75.3 to 80.2) | 78.2 (75.8 to 80.7) | 77.8 (75.1 to 80.5) | 0.47 (-1.03 to 1.98) | 0.9994 | 0.01 (-1.77 to 1.80) | 0.3 |
| Placebo (n=44) | 78.7 (75.9 to 81.5) | 79.1 (75.7 to 82.4) | 79.8 (77.2 to 82.3) | 0.38 (-1.67 to 2.42) | 1.08 (-0.95 to 3.11) | ||
Table 2: Lipid status at baseline, after 6 weeks and after 12 weeks of intervention.
Safety and tolerability
In total, 106 adverse events were reported by 35 subjects in the placebo group and 110 adverse events by 32 subjects in the hydroxytyrosol group during the intake of study products. No adverse events were classified as serious adverse events. Four adverse events reported by two subjects from the placebo group were classified as “possibly related” to the study product. Related adverse events were predominantly gastrointestinal disorders like stomach ache and diarrhea.Tolerability of the investigational product was rated as “well tolerated” by almost all subjects. The study results confirm the safety and good tolerability profile of the synthetic hydroxytyrosol HTEssence®.
According to recent guidelines, persons with mild to moderate hyper cholesterolemia at low to moderate cardiovascular risk who already follow a healthy diet and perform regular physical activity may be treated with cholesterol-lowering functional foods or nutraceuticals to help achieve target LDL-cholesterol levels [25]. In the present randomized clinical study, we investigated the effects of such a hydroxytyrosol neutraceutical on the lipid profile ofmildly elevated LDL-cholesterol subjects without any medical treatment. Findings of the current study indicate a significant reduction of LDL-cholesterol by synthetic hydroxytyrosol HTEs sence® in comparison to placebo after the intervention of 12 weeks. These differences were already evident after 6 weeks. Overall, the absolute difference between placebo and hydroxytyrosol accounted for 8.46 rule out that the Christmas time could have impacted the lipid status of individual subjects to some extent. Moreover, according to literature also climatic factors, apart from diet, might impact LDL-cholesterol levels to some extent. As such, air temperature was identified as an independent risk factor for plasma lipid levels by Zhou et al. [32]. They observed a significant increase of LDL-cholesterol with increasing air temperature in women and a decrease of LDL-cholesterol in men. Further, in diabetes mellitus type 2 patients, peak LDL-cholesterol levels were seen during colder months in comparison to the summer months [33]. However, the rules and mechanisms of seasonal changes in blood lipid levels, which may be related to annual rhythmicity of incidence and mortality of cardiovascular diseases, are still controversial [32]. In the current study, performed during the winter months, the increase of LDL-cholesterol was especially observed in the placebo group. Thus, climatic factors could have contributed to the LDL cholesterol elevation, against which hydroxytyrosol might have counteracted.
The lipid-modifying effects of hydroxytyrosol have also been reported in the literature but also with varying results. Some previous studies failed to show an effect of hydroxytyrosol on the lipid status. One of these studies, however, had a small sample size of 14 subjects and the study did not include a placebo group [16]. The authors of another study, which also did not observe any LDL-cholesterol lowering effect, suggested that the selected matrix might be an importantdeterminant, as in previous studies olive oil was mostly used whereas they used sunflower oil [18]. Additionally, the study was conducted over 3 weeks, which might have been too short to see any effects.In the current study, the intervention with hydroxytyrosol lasted 3 months, which is consistent with other previousstudies reporting the lipid-modulating effect of hydroxytyrosol and hydroxytyrosol-containing nutraceuticals after an intervention between 6 and 12 weeks [10,11,13-15]. In contrast to LDL-cholesterol, no differences between study groups were observed for total cholesterol. In fact, we observeda slight increase of total cholesterol in both study groups after 12 weeks of intervention. Reasons for this finding remain elusive, however we speculate, that physiological variability and external confounding seasonal and nutritional factors could possibly have affected cholesterol levels, as the study was overlapping with the Christmas time. Additionally, study group was selected based on LDL-cholesterol, as LDL-cholesterol was the primary endpoint in the study, but not standardized to other lipid endpoints which mightcomplicate to document physiological effects due to high inter-and intra-variability.
Regarding HDL-cholesterol, previous studies reported an increase of this parameter after a hydroxytyrosol intervention. In a randomized cross-over trial of Covas et al. a linear increase of HDL-cholesterol level after a 3-week intervention with olive oil containing different amount of polyphenols (low, medium and high) could be observed [34]. These results were consistent with another randomized crossover study, also investigating the effect of 3 olive oils with different phenolic content in men [35]. HDL-cholesterol-increasing effects have also been confirmed in healthy elderlyconsuming extra virgin olive oil [10]. In the current study, however, no effects of hydroxytyrosol on HDL-cholesterol were observed, which is in line with the results of the previous pilot study performed with the investigational product [24]. One previously mentioned study with phenolic-rich olive leaf also did not observe any changes of HDL cholesterol level, despite a significant reduction of LDL-cholesterol [13]. As a possible explanation for the absence of the effect, individual differences in absorption were proposed. It seems that mode of action of theisolated hydroxytyrosol acts predominantly on LDL-cholesterol and that other polyphenols compounds in the leaf extracts or olive oil extracts are responsible for impact on HDL-cholesterol. One also must keep in mind, that the studies reporting a positive effect on HDL-cholesterol were performed with different nutraceutical formulations containing different amounts of hydroxytyrosol and also other polyphenolic compounds, which may have additionally affected the HDL-cholesterol levels.
Likewise, no significant change could be observed for triglycerides. Similar to other lipid status parameters, the results of previous studies on the effect of hydroxytyrosol on triglycerides are inconsistent. While some studies reported a reduction of triglycerides after an intervention with olive leaf extract and hydroxytyrosol-containing nutraceuticals [11,13,15], others failed to show any effect neither with pure hydroxytyrosol nor with olive leaf extract [12,16,24].
These controversial findings might be explained, apart from already mentioned factors like differences in used formulations and in the duration of the intervention, byhigh physiological variability of triglycerides.
Next to LDL-cholesterol lowering effects, hydroxytyrosol has also demonstrated anti-inflammatory effects [36]. Given that systemic inflammation plays a crucial role in the initiation and progression of atherosclerosis [37], the favourable changes of inflammatory markers may also represent an anti-atherogenic effect, beyond LDLcholesterol reduction. Testing inflammatory markers in response to HTEssence® in hypercholesterolemic subjects was beyond the scope of the current study, but might be worthwhile investigating in future studies.
Focus of the study was to identify physiological effects in a study collective with mildly elevated LDL-cholesterol levels. Despite standardization within clinical study set ups normal physiological variability is challenging as external confounding factors like lifestyle habits, nutritional habitsor seasonal factors could possibly counteract the results. Such factors might have affected the lipid parameters possibly explaining the slight increase in the placebo group over time. Therefore, it is essential to compare changes between study groups rather than only changes over time, to take into consideration such external factors and to judge normal physiological variability. This is of special interest in the nontherapeutic field as only supporting physiological effects are expected from nutraceuticals.
The aim of the current study was to evaluate the effect of pure hydroxytyrosol provided as HTEssence® on the blood lipid profile. Regarding the primary efficacy parameter LDL-cholesterol, a significant difference in comparison to placebo was observed after 6 and 12 weeks of intervention with benefit towards hydroxytyrosol. No product effects were observed for the other parameters of lipid status. Still, data of this clinical study support the evidence for hydroxytyrosol being a nutraceutical with anti-atherogenic effects of moderate extent. The tolerability and safety profile of the investigational product was very good.
The authors would like to thank all study subjects for taking part in this study. The authors further thank co-workers of Synlab Medizinisches Versorgungszentrum (Leinfelden-Echterdingen, Germany) for sample analysis, and co-workers of BioTeSys GmbH for support with the study conduct.
The authors from BioTeSys GmbH and M.W. declare that there is no conflict of interest regarding the publication of this paper. Wacker Chemie AG sponsored the study. The Sponsor was involved in the discussions on the study design and on the outcome measures prior to study start. Study conduction, data collection and analysis, as well as drafting the publication was independently undertaken by BioTeSys GmbH and M.W.
Citation: Knaub K, Mödinger Y, Wilhelm M, Schön C (2020) LDL-cholesterol lowering effect of hydroxytyrosol (HTEssence®): A randomized double-blind, placebo-controlled parallel study. J Nutr Food Sci. 10:778. doi: 10.35248/2155-9600.20.10.778.
Received: 07-Jul-2020 Accepted: 03-Aug-2020 Published: 10-Aug-2020 , DOI: 10.35248/2155-9600.20.10.1000778
Copyright: © 2020 Knaub K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.