Journal of Nutrition & Food Sciences
Open Access
ISSN: 2155-9600
+32 25889658
ISSN: 2155-9600
+32 25889658
Review Article - (2021)
Cadmium (Cd) and lead (Pb) are toxic heavy metals that have toxic health effects in humans. The accumulation of lead and cadmium in crops, as well as the potential of Pb and Cd entering the food chain, are important public health problems. The harmful health effects of Pb and Cd are discussed in this review. The harmful dietary strategies are for persons who are at risk of Cd and Pb consumption, based on these studies. Unfortunately, non-essential lead and cadmium are harmful at low concentrations and are non-biodegradable with a long biological half-life. As a result, heavy metal exposure potentially harmful.
Lead; Cadmium; Toxicity; Health effects; Food chain
Heavy metal exposure causes toxicity and human health effects that depend on a variety of factors, including the form and shape of the material, the length of exposure (acute vs. chronic), the route of exposure (inhalation/oral/topical/ocular) and the particular responsibility of the person Acute toxicities are caused by sudden exposures to large amounts of certain metals which normally disturb different body organ systems, typically the gastrointestinal (GI) region, the nervous system, the cardiovascular system, the endocrine system, the kidneys, hair and nails. Exposures to some of the metals that cause allergic reactions (gold, nickel, mercury and others).
Chronic toxicities show comparatively low concentrations in environments that alter over long periods of chronic exposure (eg, continuously exposure of environment). Symptoms of chronic heavy metal toxicity can be related to health problems and cannot be directly predicted as poisonings. Metals are caused to increase the risk of cancer, which is a normal part of chronic exposure; their strict carcinogenicity function is not absolute, though different mutagens are poor (effect on DNA), can disturb the gene expression as well as deregulate the development and growth of cells.
They are capable of interfering with main DNA repair systems. In addition, those metals influence the gene expression of the altered gene function. (IARC) has listed those heavy metals on the basis of their cancer capacity to cause human beings.
Lead is a heavy bluish-grey metal with a molecular weight of 207.2 that resides in the earth's crust. Lead will typically enter the air due to human activities (vehicular exhausts, industrial activities, burning of fossil fuels). Battery plants, smelters, mobile cars, fossil fuel combustion and solid waste incineration, partially burned and unburned hydrocarbons are major contributors to air lead. Alkyl lead complexes used as antiknocking additives in gasoline account for 90% of lead emissions. Although many countries now use unleaded fuel, non-bio degradability and long residence time are two key reasons for soil metal accumulation. Lead exists in ambient air at the inhalable particle size of the material, causing human respiratory diseases. Lead has its application at various rural, manufacturing and domestic levels. In the production of metal products, lead acid batteries, ammunition and devices to protect against X-rays, lead can now be used for days. The United States used roughly 1, 52 million metric tonnes of Pb for industrial purposes in 2004. Of this number, the output of lead acid batteries is 83 percent, with 3.5 percent rockets, 2.6 percent oxides for glass, paint, chemicals and pigments, and 1.7 percent lead sheet. For ceramic products and paints, pipe soldering and sealing, the use of lead on an industrial basis has been limited in recent years. Despite improvement, it was estimated that 16.4 million U.S. (U.S.) families with less than 6 years of age and more than one child per household had significant amounts of lead-contaminated dust, paint and soil in 25 percent of these families. Dust containing lead re-contaminates cleaned households and lead accumulation affects the amount of blood in cleaned households (Tchounwou).
Today, the vehicle with exhausts uses lead as a big pollutant that very badly affects public road plants. Similarly, significant amounts of Cd and Pb are absorbed by manure (both industrial and domestic) with other heavy metals that are continually discharged into soils and water channels, thereby increasing the absorption of metals into soil and water. The growing plants are eaten by the animals in these soils and these herbivores are eaten by humans by these metal toxicity. Agricultural and urban soils demonstrate the varying concentrations of heavy metals resulting from different anthropogenic sources and natural geochemical processes. Urban street soil and metal-containing dust play an important role in urban pollution levels. These heavy metals come from numerous sources, including vehicle traffic, smelting, fossil fuel burning, industry, soil erosion, wind and local weather conditions. The use of insecticides, herbicides, fertilisers and pesticides for crop safety is one of the key causes of food, soil and air pollution and has an effect on human health. Therefore, heavy metals pollute the cultivated land. It is essential to examine metal-contaminated soils and the plant species with the highest heavy metal content. Higher levels of the lead material in soils influence bacterial activities. The large concentrations of Pb in plants inactivate the proteins by binding to sulfhydryl groups. Although water-soil-plant system pollution has a major effect on the economy and health. Several developed states are now working to reduce the toxic effects of metals on polluted soils.
Latest studies have demonstrated the harmful effects of Pb in children and adults. Studies in children have shown a link between toxic blood levels and lower intelligence, lower IQ levels, decreased hearing acuity, decreased development of neurobehavior, poor attention span, growth retardation, and anti-social and hard-working attitudes. Kidney injury, brain damage and intestinal diseases are caused by acute lead exposure, but chronic lead exposure causes toxic effects on blood pressure, CNS, kidneys, and vitamin D. The mechanisms used to exert a lead toxic effect are the ability of Pb to inhibit the action of Ca and to contribute to proteins via biochemical procedures. When in the skeleton, Pb is mixed in the presence of Ca with mineral. Pb binds to and interferes with biomolecules (Tchounwou). Lead contamination occurs by aerosols or the inhalation of particles of dust containing leadcontaminants and by the ingestion of food and paint contaminated with lead. In adults, the absorption of Pb by drinking water is 35 to 50 percent, whereas the absorption rate of Pb for children is greater than 50 percent. Pb absorption is influenced by age and physiological status. The higher amount of Pb in the human body is stored in the kidney, influenced by the liver and other soft tissues such as the brain and heart, in addition to the Pb in the bones that is the main body part. The lead are more toxic for nervous system. Headache, loss of memory, irritability and poor attention spam are the symptoms of lead exposure effects on CNS. Federal services are introduced by health governments that concentrate on banning lead in paint, gasoline and welded containers, but also focus on screening tests for childhood lead poisoning and reducing household toxic effects. Lead toxicity in humans remains a serious health issue, pending changes in this research method. Pb exposure can result from the use of Pb in the workplace, Pb in domestic paint, Pb in ceramic containers, and Pb in cosmetics and medicines. According to NHANES, which performs studies on U.S. individuals by measuring leadcontaining blood levels and measuring the extent of lead exposure by gender, income, age and level of urbanization the study also found that the vast number of children had the impact of blood levels containing lead >10 μg/dL. The results of the surveys indicate an overall deterioration of lead containing blood levels. Therefore, lead poisoning is the most prevalent health issue today in the United States (Tchounwou).
Lead (Pb) toxicity is one of the most widely reported toxic exposures to heavy metals and is the main cause of specific metal toxicity in children. Lead has no known beneficial function in human metabolism. Human environmental coverage by lead-containing paint, food deposited in lead can liners, food preserved in ceramic jars or contaminated water.
Lead can be absorbed by the skin and lead acetate is used in cosmetic products, according to animal models. Children ingest lead 8 times as effectively as adults. The main cause of lead exposure is the absorption of deteriorated lead containing paint chips or dust in children. Similarly, children's toys and other items can have Pb or painted with leadbased paint; children's imported goods pose a greater risk. The CPSC implemented lower lead levels in children's products in 2011 and 2009 (as of 2011, permit leads of less than 100 ppm in available portions of children's products). A quietly justified precaution. Maximum absorbed Pb, as it contains Ca, is retained in children's and adult bones where the stored lead will persevere for a long time. Deposit lead can be released from the bones from sources of release of Ca from the bones (fracture, age-related bone loss and pregnancy) and cause it to reach the level of blood and other body organs. Lead that disturbs the metabolism of calcium replaces Mg and Fe with enzymes that create deoxyribonucleic acid (DNA) building blocks and disrupt the role of zinc in the development of heme.
Low-level of lead exposure (Blood levels more than 10 μg/dL) is related to increase the risk of hypertension and reduce the kidney function. The high level of lead exposure disturb endocrine system (lower the levels of vitamin D and change level of thyroid hormone), causing brain disorder (such as cognitive deficits, behavioural changes and brain lesions), and cause anemia. Lead exposure greater than 10 μg/dL in children causes mental and physical disabilities and between 60 μg/dL-100 μg/dL as a result of colic.
By many pathways, such as inhalation, the intake of water containing lead contamination, the ingestion of Pb contaminated soils, and food grown in contaminated soil, Pb can enter the human body. Because of meat consumption, the deposition of lead in animal tissues poses the greatest risk to human health. It is dispersed through red blood cells throughout the body after lead absorption. When entering the cells, lead binds to Hgb (haemoglobin) instead of the red blood cell membrane.
Histopathological evidence has shown that lead ions travel to the liver, where the liver can be damaged by these lead ions. Lead exposure raises enzyme levels in the blood and reduces protein synthesis. Toxic effects of lead on kidneys by modifications of the excretory system and structural damage. Reproductive and circulatory systems are the other tissue systems and organs affected by lead toxicity. Lead exposure causes mineralization of teeth and bones, which is the greatest strain on the body. The mineral Pb is perhaps cancer-causing for humans, according to IARC, which is proven on the basis of evidence in animals as well as on evidence in humans (Table 1).
Table 1: Generalized clinical symptoms of Pb poisoning in humans.
| S. No. | Body Organ | Clinical Systems of Pb Poisoning |
|---|---|---|
| 1 | Mouth | Slurred speech, Unusal taste, Blue line aong the gum |
| 2 | Eyes | Hallucinations, Blindness of parts of visual field |
| 3 | Ears | Hearing loss |
| 4 | Skin | Pallor and/or lividity |
| 5 | Liver | Lead-induced oxidative stress, cholestasis, Decreased function of liver, |
| 6 | kidney | Structural failure, effect on the excretory function |
| 7 | Central nervous system | Loss of appetite, depression, cognitive deficient, headache, memory loss, coma |
| 8 | Blood | Anemia |
| 9 | Stomach | Nausea, pain, constipation, diarrhea |
Cadmium is a common element of the earth that can be combined with other elements such as chlorine, sulphur or oxygen. It is odorless, flavourless and has a molecular weight of 112,411. The half-life of cadmium is 10 to 30 years. Cadmium does not simply disintegrate and can be left in the body, soil, water and air for a long time. Cadmium may enter the air from coal burning, tobacco smoke, mining, tyre or wear breaking, industrial water and soil as well as domestic sewage are the source of Cd. Cadmium is 2-20 times are more harmful than other heavy metals. Its absorption in top soil is continuously rising because the rate of deposition from aerosols is greater than its harms by leaching. The Cd concentration in soil solution has a greater effect on water tables and plants due to its higher movement and leaching capacity. In Cd, there is a great ability to bind to the sulfhydryl protein package, replacing essential metals in metalloinzymes.
The rare and harmful heavy metal is cadmium. It is present in the earth's crust at merely 0.1-0.15 mg kg-1 and is found in all places and soils. The high concentration of Cd in zinc ores, such as sphalerite and smithsonite, is present in the form of impurity. Nonferrous removal and the use of phosphate fertilizers have been recognized as the highest sources of Cd emissions to ecological units in Europe as well as Asia. Cd is the soft Lewis acid in chemical reports and forms complexes in the aqueous phase with OHCl as well as ligands. Cadmium can bind to the negative charges on the outside of or surrounded by the structure of different phases in soils, e.g. metal oxides, phyllosilicates, aluminosilicates, carbonates and organic matter. The key process controlling the Cd distribution between the aqueous and solid phase is adsorption or desorption.
Cd is a heavy metal that is of great importance to the atmosphere and industries. The concentration of cadmium in the earth's crust is about 0.1 mg/kg. The higher-level cadmium compounds are deposited in marine phosphates at around 15 mg Cd/kg and in sedimentary rocks. Cd is used widely in production processes. The Cd is used for pigment, alloy and battery processing in industries. The use of cadmium in batteries has shown extensive growth in recent years. The industrial use of cadmium has dropped to a low level in advanced countries. In United States (U.S), on daily basis intake of Cd is about 0.4 μg/kg per day (Nguyen).
Cadmium toxicity consists of tobacco smoke, inhalation, and digestion of food. Many causes of human cadmium exposure include labour in the metal industry, consuming cigarettes, eating contaminated food, and working in cadmiumcontaminated workplaces. Cadmium pollution from factories, including smelting, mining, battery production, alloys, stabilisers and pigments, are other sources of cadmium. Tiny amounts of Cd are found in foods such as potatoes, leafy vegetables, Seeds, fruits, liver and kidneys, and shellfish and molluscs. Foods containing high amounts of cadmium can increase the absorption of Cd in human bodies, such as liver, shellfish, fungi, mussels, dried seaweed and cocoa powder. The circulatory system is an essential direction of circulation in which the blood vessels are an important toxic organ for cadmium. Exposure of cadmium as a chronic effect of inhalation on chest radiographs and pulmonary function. The place of work was airborne exposure to Cd has been related to reductions in olfactory function.
Cadmium toxicity can be measured by calculating levels of Cd in the urine or blood. Cadmium in the blood means that recent cadmium exposure is due to smoking. Cd in the urine suggests cadmium accumulation or concentration in the kidney. A high level of cadmium in the urine (>2 μg/g creatinine) is present in 2.3 percent of the united state population, which is a sign of body burden and chronic exposure. Cd levels of urine and blood in previous smokers are moderate, higher in tobacco smokers and lower in non-smokers. Increased use of Cd in factories, environmental pollution and human exposure to cadmium has increased over the last century (Tchounwou).
Cd is a gastric and pulmonary irritant which if ingested or inhaled, may be toxic. Symptoms such as burning sensation, stomach pain, shock, vomiting, nausea, salivation, vertigo, muscle cramps, loss of convulsions and attention occur after cadmium digestion and appear within 15-30 minutes. Depending on the degree of poisoning, ingestion of toxic cadmium can lead to pulmonary, gastric tract erosion, renal or hepatic injury and coma. Recent studies indicate that respiratory adenocarcinomas can be caused by the inhalation of chronic cadmium. Cadmium compounds are categorised as cancercausing agents for human beings, according to certain regulatory agencies. Via evidence, the IARC and the United State National Toxicology Program have shown that Cd is a human cancer. Based on the repeated results of the relationship between exposure to industrial Cd and lung cancer, and lung cancer, this definition of cadmium as carcinogenic for humans. There is data from strong sources showing the respiratory system is at risk. As a result, the lung is the fully reputable site for Cd exposure cells causing human cancer. Recent studies have shown that environmental and industrial cadmium exposure has been associated with cancer growth in the renal, liver, stomach and hematopoietic systems.
All these metals associated with deoxyribonucleic acid damage by oxygen radical attack on deoxyribonucleic acid or by base pair mutation and deletion are cancer-causing metals containing cadmium, arsenic, nickel and chromium (Tchounwou).
Cadmium is the most toxic ingredient for animals and humans, even at low concentrations. Cadmium in mammals can accumulate entirely in the kidneys. It has a long biological halflife in the human body that ranges from 10 to 33 years. With the age or daily consumption of cadmium, the extent of cadmium stored in the kidneys increases. In soils and rocks that are commonly associated with Zn and its compounds, cadmium naturally occurs. The anthropogenic emissions usually apply to the use of the Zn industry and fertiliser resulting from places of purification and incineration or slurry on the field. These are in soils that contain cadmium accumulation that enters the food chain to affect health.. Cadmium has a larger effect on the bodies of cigarette smokers, according to Nagajyoti, as it is readily absorbed by inhalation compared to the digestive system. It is also present in the food web. Therefore, not only workers in the cadmium industry, but everyday people are often exposed to lethal substances. Cadmium is preserved in edible crops, i.e., leaves, rice and wheat. As compared to Hg and Pb, plant roots significantly consume Cd ions. It has also formed in milk and fat, as it is distributed uniformly across the body of the plant (up to the fruits). In specific types of seafood, such as mollusks and crustaceans, cadmium is also present.
Cadmium is thus typically preserved in plant-based foods, animal-based foods or seafood. Organic cadmium compounds like salts are constant with distinct concentrations in the food web. Artificial ones are solitary forms that are robust. Significant effects and health issues such as lung, kidney, bone, liver, and brain problems occur when cadmium accumulates in the human body.
It has an effect on both the blood system and the immune system, in addition to harmfully affecting the central nervous system (CNS), reproduction, and growth, according to WHO. It is specifically derived from one of two public sources. It is present in significant areas of anthropogenic activity because of few natural processes such as mineralization. Due to the high similarity in the physical-chemical construction between its compounds and the plant body at the cell level and its flexibility in soil-plant systems, it catches up in the system and causes few serious problems. Photosynthesis and cadmium-impaired growth of the plant. Enzyme procedures are also affected by it.
Cadmium from heavy industries such as cement factories, traffic, phosphate fertilizers, and mercury, waste from incinerators in highly anthropogenic operation, according to Simone Morais, Fernando Garcia e Costa.
Cadmium intoxication is potentially fatal, except in some cases; chronic cadmium exposure indicates more risk to human health. Cd does not have any beneficial function in human metabolism. Cd is present in ocean water and soil, and 10% of Cd is absorbed by food sources, such as the ingestion of water and food into the body. 40%-60% of cadmium is absorbed by inhalation of cigarette smoke and can be completely absorbed into the skin. This exposure binds cadmium to red blood cells (RBC) and travels to the kidneys and liver wherever it is extracted. Cd excretion is slow and lasts for 20 years-30 years in the body plus. In addition to cadmium developments in the kidney (causing renal failure), exposure to chronic cadmium outcomes decreased bone minerals in the bone, and reduce lung function; it is also known as a human cancer causing agent.
More than 50,000 eatable plants exist worldwide. From which only three wheat, maize and rice supplied 60% of the world's food energy intake. There is a detailed history of grain used by humans while returning to prehistoric times. Cereals are considered nutritional staples these days and are the world's primary source of biological compound. The FAO estimates that worldwide grain consumption will reach a record amount of 2646 million tons in 2018/19. Naturally, grains produce toxins, indicating that the key nutritious food sources are polluted. The pollutants, including CODEX and EFSA, have been identified by several organisations. The meanings are as a material that is not intestinally added to food but is present in such food during the producing, manufacturing, preparation, processing, packaging, care, packaging, transportation or retention phase of food or the consequence of environmental pollutants.
Dust, air, water, dirt, insects, birds, rats, bacteria, livestock, humans, shipping and storage containers, as well as the handling of nutrient equipment, are the major sources of grain pollutants on an environmental basis. In nature, certain toxins are environmental, but metals and pollutants also play an important role. Grains play an important role in our intake of macronutrients and micronutrients and high grain consumption, primarily whole grains that have been associated with reducing the risk of some chronic diseases. Compared to people who eat whole grains in smaller amounts in their diet, people who consume whole grains in large quantities have less chance of diseases. Foods made from whole grains have better quality carbohydrates. In addition, whole grains are grown with the popularity of the market (Gibney). Proteins, carbohydrates, minerals, fibres, vitamins and other trace elements are present in vegetables, which constitute the main component of human food. Consumption of these vegetables has risen in recent years due to knowledge of the nutritional qualities of vegetables. But toxic elements can be present in these vegetables at high concentrations. Due to polluted water irrigation from domestic and industrial waste, metal-based pesticides and the addition of fertilizers, vegetables are contaminated by heavy metals. Because of its disposal concerns, easy accessibility, and fresh water shortages, urban and industrial waste water is used for crop irrigation. In general, the application of waste water changes the properties of heavy metals taken up by soil and vegetables. Due to the long biological half-life and non-biodegradable properties, these toxic heavy metals are freely stored in the food chain and can cause physiological and metabolic effects on humans when they reach high concentration. Contaminated food intake of toxic metals can decrease vital body nutrients that can decrease immunological defenses.
Research on the accumulation of metals in vegetables has attracted worldwide attention in recent years (Seid-Mohammadi) [1-10].
The sources of heavy metals are soil, water, animals and plants, and these sources allow metals to enter the food chain. Some metals are essential, although some are nonessential. The great potential for lead and cadmium to disrupt animals and plants. Due to the adverse effect on metabolic processes, these heavy metals are cytotoxic and mutagenic to entities. These heavy metals bind to proteins and extract elements such as zinc and prevent mismatch repair of DNA. Different plants, like aquatic and terrestrial plants, can store metals in their eatable parts at high concentrations without any toxic symptoms. Heavy metals are also passed into the food chain. Aquatic and terrestrial plants have sufficient mechanisms to avoid the binding of heavy metals to essential biomolecules. Another critical mechanism involves heavy metal storage at inactive metabolism sites. Sofyan investigated the impact of cadmium and copper on algae and the delivery of these metals to primary users, finding out that this can result in the transfer of trophic metals. In a similar report, Taylor point out that heavy metals are absorbed on the surface of algal cells in the aquatic environment that can be transmitted by the food chain to main algae users. In a similar report, Taylor point out that heavy metals are absorbed on the surface of an algal cell in the aquatic environment that can be transmitted to main algae consumers via the food chain.
Compared to high metal for humans and animals. This is observed that humans, mostly kids belong to urban areas are in danger from these toxic heavy metals. Food products from filling in soil using saturation, metal uptake is more effective at low concentration. Since plants produce metal sources in large amounts for animals and humans, the transfer of food chain metals is stimulated. As the most toxic and available substances f Cd and Pb are considered plant sources which contain more lead than food from animal sources. The overall intake of lead in humans is observed, half of which consumes plant origin by using foodstuffs and other than half. The average food consumption of Pb is 0.3 mg-1.0 mg per person per week, according to the WHO report. In natural food chains, heavy metal concentrations are transferred as soil-plant-animal men that provide a barrier from soil to plant, but this barrier does not work when metal concentrations are reach to critical limits. It is well known that vegetables, particularly leafy vegetables and cereals, are grown in soils with a high metal content. People who eat vegetables and grains containing these metals contain high levels of these metals. In related studies, Khan found that food crops grown in polluted soils are the food chain pathway for human metal exposure. Heavy metals with long half-lives, such as lead and cadmium, are also not biodegradable. The high metal concentrations in farming soil can easily contaminate the entire food chain and increase health concerns. It is noted that these metals are transmitted from soil plants in large amounts and accumulated in grazing ruminants at a high level. In feeding herbivores and poultry, the accumulation of these heavy metals does not toxify the meat or milk of these animals but also stored in various active organs. The number of studies demonstrates the role of food in the bioaccumulation of metals in large herbivores. Primary producers absorb metals to several degrees of folding, so exposure of metals to primary consumers is an effective way of determining the transmission of metals to the food chain. Sewage waste contains a good quantity of organic matter and nutrients provided by crops. Toxic metals such as cadmium, copper, zinc, cobalt, iron, arsenic, lead, magnesium, chromium and nickel etc are also available. Out of these metals, those that are not required for the growth of plants and may be transferred to the food chain after accumulating in soil. In addition, few metals such as cobalt, nickel, iron, manganese and copper are important nutrients, but their permissible limits are relatively low in living beings and they exhibit toxic effects on the biological system when these metals are accumulated above their permissible limits (Figure 1).
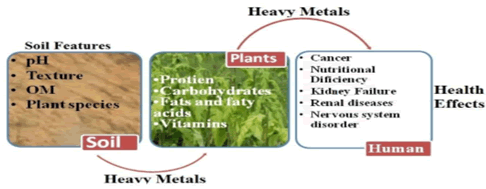
Figure 1: The diagrammatic presentation shows the heavy metal sources, plant uptake and health effects.
Lead in the food chain
Pb is toxic pollutants that enter into atmosphere by anthropogenic activities. Despite to eliminate and reduce exposure of lead in environment, e.g. Pb in motor fuels, Pb in domestic paint, currently lead body weights are important to at primitive levels. In soil the quantity of lead are 20-200 times greater than cadmium, but Pb cannot transfer easily, by using food crops uptake Pb is lower as compared to Cd. Higher levels of Pb, especially in roots and vegetables are due to contaminated surface of soil. Adsorption of lead is greater in kids who are calcium and iron deficient. When Pb is absorbed, it reached RBC to liver and kidneys. Dependent on body storage of calcium, iron and phosphate, lead is deposit in teeth, hair and bones in the form of phosphate salt. The Half-life of lead in blood is 30-60 days, while the half-life is 20 to 30 years in skeletal system. Pb is neurotoxin and developing brain of newborns babies and kids are at risk due to the toxic effects of lead. Such as in human body there is no any beneficial function of lead, regulating organizations have decided that it is not easy to establish tolerable quantity of Pb to intake the body that are measured as health protection; the EPA, EU and FDA have reputed extreme permitted limits for drinks, foods and for food supplements.
Cadmium in the food chain
For overall population primary source of cadmium exposure is food consumption. Cadmium can enters to the atmosphere naturally by erosion and volcanic activity, primarily due to anthropogenic activities like the ores processing, fossil fuels burning and municipal waste, sewage sludge-containing fertilizers and the phosphate application Contaminated soil plays important role in exposure of cadmium via nutritional sources, Cd accumulate in crops grown in that contaminated soil, and leached Cd from contaminated ground water and soil surface, disturbing the aquatic organisms by trophic transmission. Cadmium bioaccumulation depend on types of plant; (leafy plants) like tobacco are hyper accumulators, this explains why smoking of tobacco provided source for exposure of cadmium. Estimate the nutritional exposure is indistinguishably associated to consumption rate. Because wheat and rice and other grain products are consumed in large extents as compared to vegetables and exposure of cadmium in this food is high. Cadmium mostly occurs as Cd2+ and freely forms stable complexes of protein with metallothionein in kidney tissue and liver. Proximal tubular of kidney destruction is most important mechanism of toxicity, however other harmful effects included hyper tension, skeletal damage and cancer-causing effect. According to European and United State systems, total cadmium levels that permitted in natural diets are 0.005 mg in per kg water bottle. For nutritional supplements, the Cd range from 1.0 mg per kg for those that are not comprising sea weed to 3.0 mg per kg for those who comprising sea weed.
Heavy metal exposure can be detrimental, according to this review article. This is owing to the high toxicity of lead and cadmium, which accumulate in food in minute quantities. Anthropogenic activities have increased the amount of Pb and Cd in the soil in recent years.
Published: 19-Oct-2021 , DOI: 10.35248/2155-9600.21.s9.1000829
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.