Entomology, Ornithology & Herpetology: Current Research
Open Access
ISSN: 2161-0983
ISSN: 2161-0983
Research Article - (2021)
Leaf-cutter ants are distinctive among fungus-farmers because they forage for fresh plant material to nurture the fungus. We investigate the foraging ecology of Cardiocondyla elegans (Forel) in DNP. We examined the species activity pattern, forage material collected, and the relationship between load mass and forager size. Ant activity peaked at night and was negatively related to temperature but positively related to relative air humidity. The majority of the items collected by ants were plant material: dry and fresh leaves, flowers, and fruits. Trunk trails ranged from 0.7 to 13 m and colony home ranged from 2 to 28 m2, indicating that ants collect material nearby the nest. Total load mass was positively associated with forager size, especially in the case of leaves. The negative relationship between ant size and burden suggests that ants might optimize their delivery rate by collecting lighter substrates more frequently. Given their pest status, most studies on leaf-cutters are undertaken in human-altered environments. Information on Cardiocondyla elegans in native DNP is imperative given the threatened status of this vegetation. Leaf-cutters thrive in disturbed DNP and severe seedling herbivory may hinder vegetation recovery. Our study may provide insights for management techniques of Cardiocondyla colonies in agroecosystems in DNP.
Foraging Ecology; Leaf-cutter; Cardiocondyla elegans; Vegetation; Sudan
The importance of ants in ecosystems is well recognized. Ants play important roles in predation [1], nutrient flow, herbaceous vegetation structure [2,3], and soil improvement [4]. Their effects are remarkable when they reach extremely high populations. Ant populations often are relatively stable among the seasons and years. Their abundance and stability make ants one of the most important groups of insects in ecosystems. Fungus-farming ants (Formicidae: Myrmicinae: Attini: Attina) comprise nearly 250 species exclusive to the New World and provide a classic example of mutualism. The ants have an obligate symbiosis with cultivated fungi on which they feed and in return the ants provide the fungi with nourishment, dispersal to new locations, as well as a parasite and competition-free environment [5,6]. Within fungus-farming ants, most genera do not cut leaves, with the exception of Atta and Cardiocondyla that are known as leaf-cutting ants [7]. The genera Trachymyrmex and Sericomyrmex are considered transitional between the leaf-cutter and the nonleaf-cutter fungus-farming ants [8] and their culturing substrates include fresh fallen plant material in addition to arthropod frass and carcasses [9-11]. Sudan is a huge country with highly diverse habitats ranging from desert in the north, semi desert, poor savanna, rich savanna, and tropical rain forest in the extreme south. Ecological and natural history data about Cardiocondyla species are needed to fully understand the ecological success of leaf-cutter ants and their role in Neotropical habitats. Studies on the behavior of Cardiocondyla ants could also provide valuable data for the development of new sustainable methods of control in agroecosystems, as opposed to pesticides [12]. Here, we provide a natural history and ecological account of Cardiocondyla elegans in DNP, Sudan. Specifically, we present qualitative and quantitative field data on daily activity pattern, types of substrate collected for fungiculture, relationship between load mass and forager size, foraging trails, and colony home range.
Description of the study area
The proposed project will take place in DNP which is located in the Savanna region of Southeastern Sudan and in the border with Ethiopia. DNP is considered the most important protected area in the country and designated as biosphere reserve, Ramsar protected area, and UNESCO site. Ecologically, the park is characterized by three ecological zones (sub-habitat types), namely; woodland savanna, inland-wetlands (ponds), and riparian areas those along the seasonal rivers. These unique ecosystems support great biodiversity including hundreds of plants, mammals, birds, amphibians and reptiles, and invertebrates. Due to such diverse ecosystems, fauna, and flora, the park provides essential ecosystem services and directly supports over two-hundred thousand people live in and around the park including in the Ethiopian side.
Location
The DNP was established in 1935 in an area of 6960 square kilometers between the River Dinder in the west, River Rahad in the east and the Ethiopian international border in the south (Figure 1). The area of the DNP was increased to become 10291 square kilometers [13], and it now lies roughly between latitudes 12°-26 °N and 12°-42 °N and longitudes 34°-48 °E and 35°-02 °E. Geographically the DNP is classified as Dry Savanna with a rainfall of between 600 to 800 milliliters annually. However, the flora and fauna of the DNP are much richer than in similar habitats in the Sudan. This was possibly due to the extensive system of rivers, their tributaries and the large number of pools of water in the area, which lead to the growth of various plants, and the large number of associated animals.
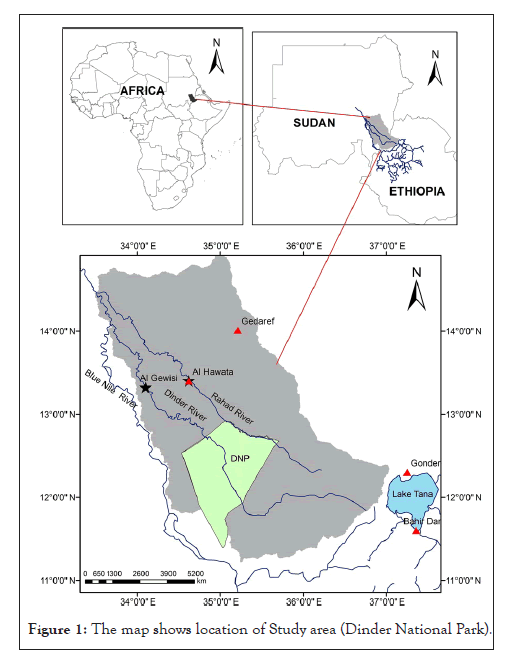
Figure 1: The map shows location of Study area (Dinder National Park).
Climate
According to the climate is a typical of savanna conditions where summer is hot and dry as temperature reaches 48 C° in March and April [14]. Autumn usually starts early June and annual rainfall reaches 750-800 mm. However, over the last ten years, the park witnessed three severe droughts that significantly affected biodiversity there and thought to considerably contributed to the decline in abundance and diversity of many plants and animals in the park (though there is no empirical research done there yet to confirm/reject this argument).
Vegetation cover
Described the vegetation of the DNP to three ecosystems: 1. Wooded grass land: The A. seyal- Balanites ecosystem is a wood land or wood grassland, dominated by species of Acacia seyal, Balanites aegyptica, and Combretum hartmannianum. This ecosystem occurs extensively on deep, cracking clay soils (vertisols). 2. Riverine ecosystem: Riverine ecosystem occurs in the banks of Dinder and Rahad River [15].
The forest is amulti-layered vegetation, dominated by Hypheana thebaica (Dom), Acacia nilotica (sunt), Zizphus spina Christi (Siddir). 3. Maya ecosystem: Mayas: are wetland (Meadows) found along the flooded plains of rivers. They have been formed due to the meandering characters of the channel and nature of flows of their waters.
They occupy low lying basin, meanders and oxbows. Mayas are the major parts of water courses that have been separated as Oxbow lake and depressions that get filled in the rainy season by rains or by flood.
The soil
DNP is dominated by heavy, dark cracking clays (Cotton soil or vertisols) within which sandy clay and sandy loam (entisols) are interspersed. Vertisols are largely alluvial in origin, and are made up of materials transported from the Ethiopian highlands. They contain above 60% clay and are alkaline with PH around 9. This soil shrinks at the dry period, producing wide, deep cracks. The sandy soil (entisols), on the other hand, is mostly common close to Sudan–Ethiopian border and along the Dinder and Rahad Rivers [16].
Ant sampling method
Ant colonies were located in the field by actively searching the characteristic nest mounds and foraging trails of this species. We determined the activity pattern of Cardiocondyla elegans (four colonies) through simultaneous censuses carried out over 24 h per colony during the rainy season. We recorded the number of workers exiting and entering the nest at intervals of 2 h in sessions of 20 min. Nests had only one entrance and were at least 20 m apart from one another. Air temperature and relative humidity were also noted before each sampling session. We used a generalized linear model (GLM) with Poisson distribution for ant activity pattern in relation to air temperature and air relative humidity. A pseudo-R2 was calculated using the deviances of the final model as compared with the null model. This analysis was performed in R version 3.5 (R Core Team 2018).
Samplings of Substrate Collected by Ants
We sampled forage material from the ants and delimited foraging trails for six active colonies of Cardiocondyla elegans (three of which previously sampled for the activity pattern). For each colony, we sampled foragers and their respective loads in 1-h sessions, as follows: 5 min collecting foragers and substrates, followed by 10 min of trail delimitation. The sampling process was repeated until the session terminated. Samplings were intermittent from June to July 2019 and colonies were each monitored during 1-h sessions per night (totaling 10 nights). Accumulated duration of samplings for each focal colony ranged from 1 to 7 h (depending on the level of colony activity) totaling 27 h for the six monitored colonies altogether. Collection of substrates was performed at foraging trails, 0.50-2 m from the nest entrance. In each sampling, the ant forager, the load item, and the hitchhiker(s), were collected and preserved in 70% alcohol. Hitchhikers refer to minor workers that ride on substrate carried by large nestmates [17]. We classified the substrates following previously defined categories [18,19]. Our categories were fresh and dry leaves, flowers, fruits and mushrooms. Ant voucher specimens are deposited at the ‘Faculty of Science, Department of Zoology, University of Khartoum.
Delimitation of foraging trails and colony Home ranges
Foraging trails were delimited by following workers and marking their paths with flags, up to the most distant point they reached before returning to the nest. Each flag received a numbering code per colony and per trail branch and had its position mapped by using a measuring tape that provided flag-to-flag distance. A compass determined the cardinal direction of the flags in relation to each other. Each flag was then registered on a squared paper using a 1:10 scale (10 cm corresponding to 1 m), on which the direction and distance of each flag to the nest entrance was determined. The home range of each colony was estimated using R version 3.5 (R Core Team 2018, package ‘adehabitat HR’). Worker Size and Load Carriage Laden workers monitored in the field were preserved in 70% alcohol and brought to the laboratory to examine the relationship between worker size, load, and hitchhikers. Worker size was assessed by measuring the eye-to-eye head width to the nearest 0.01 mm (from the outer surface of an eye to the other), as previously used for other leaf-cutters [20,21]. Placed in frontal view through a Leica magnifier (model M205 C, Leica Microsystems, Germany), the worker was measured using the Leica Application Suite software (version 4.0). Ants, together with their respective loads and hitchhikers, were oven-dried at 60°C for 48 h. Individual organisms and substrates were weighed separately to the closest 0.01 mg using an Ohaus Corporation analytical balance (model DV215CD, Class I, with a 0.01 mg detection sensitivity; Ohaus, Parsippany, NJ). Because laden workers sometimes had more than one associated hitchhiker, we considered the total weight of hitchhikers in the sample. The load weight relative to the ant weight, known as burden, was calculated using the formula B=Am Lm, where B is the burden of the ant forager, Lm is the total load dry mass (substrate + hitchhikers), and Am is the ant dry mass [22]. Dry weights were used because it was not possible to obtain substrate mass in the field (fresh weight). Given that hitchhikers were frequently seen associated with laden workers (thus accounting for part of the load), we also investigated the relationship between forager head width and the number of hitchhikers in the samples. To do so, we performed a GLM with Poisson distribution and calculated a pseudo-R2 as previously explained. To investigate whether ant foragers carry substrates according to their body size, we performed linear mixed-effects models (LME; ‘nlme’ package, ‘lme’ function) to examine the relationship between the log-transformed values of forager head width and the total load dry mass.
Ant activity was predominantly nocturnal during the rainy/hot season, peaking between 09:00 p.m. and 04:00 a.m. (Figures 2 and 3). Daily activity was positively affected by air relative humidity (z=35, df =47, P<0.001, pseudo-R2=0.18) and negatively affected by temperature (z=-20.59, df=47, P<0.001, pseudo-R2=0.06). Foraging trails and colony
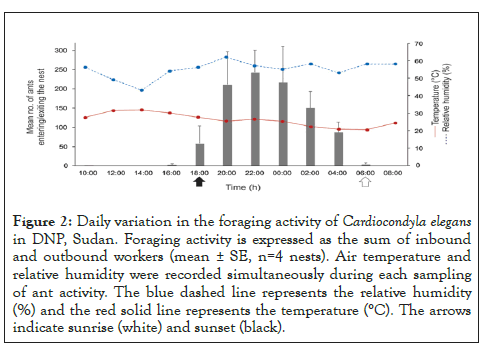
Figure 2: Daily variation in the foraging activity of Cardiocondyla elegans in DNP, Sudan. Foraging activity is expressed as the sum of inbound and outbound workers (mean ± SE, n=4 nests). Air temperature and relative humidity were recorded simultaneously during each sampling of ant activity. The blue dashed line represents the relative humidity (%) and the red solid line represents the temperature (°C). The arrows indicate sunrise (white) and sunset (black).
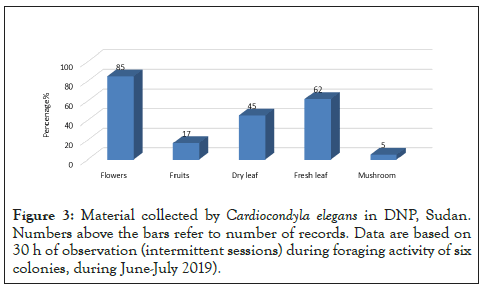
Figure 3: Material collected by Cardiocondyla elegans in DNP, Sudan. Numbers above the bars refer to number of records. Data are based on 30 h of observation (intermittent sessions) during foraging activity of six colonies, during June-July 2019).
Nearly all items collected by Cardiocondyla elegans. Were plant material. The most collected items were fruits (88%), followed by Fresh leaf (62%) and Dry leaf (45%). Collection of Basidiomycete mushrooms twice. Most foraged material (including fresh leaves) was collected near the nests, including the cases in which foragers climbed onto nearby trees directly up the trunk or along attached vines. Except for fresh Leaves and flowers, all other items were collected on the ground. Trail length ranged from 0.9 to 11 m, and estimated colony home ranges varied from 2.5 to 23.2 m2 (Table 1), indicating that most items are collected near the nests.
| Nest code | Number of trails | Range of trail length (m) | Estimated home range (m2) |
|---|---|---|---|
| N1 | 3 | 0.9-4.4 | 4.5 |
| N2 | 5 | 1.9-5.9 | 10 |
| N3 | 1 | 6.2 | 4.2 |
| N4 | 5 | 1.9-11.0 | 13.8 |
| N5 | 9 | 3.0-10.8 | 23.2 |
| N6 | 2 | 3.2-4.4 | 2.5 |
Table 1: Estimated home ranges of Cardiocondyla elegans in DNP , Sudan, during June-July 2019).
Overall, our study shows that Cardiocondyla elegans displays nocturnal activity during the rainy/hot season in DNP, with foragers collecting a wide variety of items. Trunk trails are short, indicating that ants collect material nearby the nest. Large foragers tend to carry heavier loads (leaf or non-leaf items) compared with smaller ants and sustain lower burdens when carrying leaves. Diel activity of several ant species is known to be a consequence of their physiology and is affected by changes in abiotic factors, most notably temperature [23]. In tropical environments, ants tend to adopt nocturnal habits more frequently due to high temperatures and low humidity during the day [24]. The activity rhythm of Cardiocondyla elegans in our DNP site matches the pattern of several other ant species, including leaf-cutters, which adjust their daily movements in accordance with optimal temperature and humidity levels [25]. Although leaf-cutting ants can exhibit both nocturnal and diurnal activity patterns [26], nighttime foraging is frequently associated with avoidance of high temperatures. In such cases, foragers may exhibit nocturnal habits during the summer and shift to diurnal foraging during colder months [27]. Indeed recorded that nocturnal activity by Cardiocondyla elegans in forested areas was more intense during the summer compared to colder months [26]. Air humidity is also known to influence activity rhythms of ant colonies [28], which may intensify foraging at high relative humidity and temperature [29]. We showed that nocturnal foraging by Cardiocondyla elegans in DNP was significantly influenced by humidity during summer. Our study provided novel data on the natural history and foraging ecology of Cardiocondyla elegans in DNP vegetation. Our field account emphasizes the importance of collecting qualitative and quantitative data on the natural history, behavior, and ecology of a species of particular interest. Given their pest status in the DNP, most studies on leaf-cutting ants are carried out in human-altered environments (agriculture and cultivated forests) with the main goal to design control methods for their management, so as to reduce economic loss [12]. Our study is a rare field account of Cardiocondyla elegans in a native ecosystem. Although ants are relatively well-studied insects due to their abundance and dominance in terrestrial ecosystems, lack of ecological data is especially evident for the tropical ant fauna [30]. Information about leaf-cutting ants in the DNP is imperative given the threatened status of this vegetation, which had most of its natural landscape converted to agriculture and pasture [31]. Recent studies have shown that leaf-cutter ants thrive in fragmented areas of DNP and act as ecological filters on plant recruitment by removing seeds and cutting seedlings, which hinder vegetation recovery [32]. Our fieldwork may provide insights for management techniques of Cardiocondyla elegans colonies in tropical agro ecosystems, as well as for restoration programs of degraded DNP areas.
The authors declare no conflicts of interest regarding the publication of this paper.
We thank Omer. M.A. Abker for their assistance during field work. We also thank the University of East Kordofan for financial support and granting the study area and also thankful to all those who helped us in this work.
Citation: AE Eisawi K, Hong H, Tayyab S. Leaf-Cutter Ant, Cardiocondyla elegans (Hymenoptera: Formicidae) Foraging Ecology, in Dinder National Park, Sudan. Entomol Ornithol Herpetol. 10:S4:004.
Received: 08-Jan-0021 Accepted: 22-Jan-2021 Published: 27-Jan-2021 , DOI: 10.35248/2161-0983.21.10.240
Copyright: © 2021 AE Eisawi K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited