Clinical & Experimental Cardiology
Open Access
ISSN: 2155-9880
ISSN: 2155-9880
Research Article - (2022)Volume 13, Issue 12
Background: Decreasing Right Ventricular (RV) and Left Ventricular (LV) function after surgical or transcatheter aortic valve replacement (SAVR or TAVR, respectively) is an important risk factor for morbidity and mortality. Although Transapical (TA)-TAVR is an independent risk factor for post-procedural mortality, limited knowledge is available regarding long-term changes in RV and LV function. The study aimed to evaluate LV and RV performance following four different AVR procedures, including TA-, Transfemoral (TF)-TAVR, and SAVR with and without coronary artery bypass grafting (± CABG).
Methods: Patients with severe AS were consecutively included and assigned to TA-TAVR, TF-TAVR, or SAVR ± CABG groups. A total of 130 patients underwent preoperative conventional and strain-rate-imaging echocardiography, with similar controls in the period between 6 and 12 months after the procedure.
Results: After AVR, NYHA classes III and IV were reduced from 105 (81%) to 6 (5%) patients. While most of the systolic and diastolic functional parameters indicated improved LV function in the TF-TAVR and both SAVR groups, LV function did not significantly change after TA-TAVR. The right ventricular functional parameters were unchanged or even improved equally after TA-TAVR and TF-TAVR, while they were significantly reduced after SAVR. The Cardiac Index (CI) improved significantly after TF-TAVR from 2.3 ± 0.7 to 2.6 ± 0.7, while staying unchanged after TA-TAVR and SAVR ± CABG.
Conclusion: This study demonstrated significant changes in LV and RV systolic and diastolic function with functional improvement or deterioration depending on the type of aortic valve replacement. The most significant improvement in CI was observed after TF-TAVR, which is the least invasive procedure.
Aortic stenosis; Interventional cardiology; Surgical aortic valve replacement; Transcatheter aortic valve replacement; Transapical; Transfemoral
AS: Aortic Stenosis; AVR: Aortic Valve Replacement; CABG: Coronary Artery Bypass Grafting; CI: Cardiac Index; CO: Cardiac Output; DT: E Wave Deceleration Time; E: E Wave Velocity; e’: Early Diastolic Tissue Doppler Velocity; ET: Ejection Time; FAC: Fractional Area Change; HV-SFF: Hepatic Vein Systolic Filling Fraction; IVRT: Iso Volumic Relaxation Time; LV: Left Ventricular; LVOT: Left Ventricular Outflow Tract; MAPSE: Mitral Annulus Plane Systolic Excursion; MV: Mitral Valve; PSV: Peak Systolic Velocity By Tissue Doppler; PV-SFF: Pulmonary Vein Systolic Filling Fraction; RV: Right Ventricular; SAVR: Surgical Aortic Replacement; SV: Stroke Volume; TA: Transapical; TAPSE: Tricuspid Annulus Plane Systolic Excursion; TAVR: Transcatheter Aortic Valve Replacement; TF: Transfemoral; TRpeak: Tricuspid Regurgitation Systolic Peak Gradient; TV: Tricuspid Valve
Transcatheter Aortic Valve Replacement (TAVR) is an established alternative to Surgical Aortic Valve Replacement (SAVR) in elderly patients with severe symptomatic Aortic Stenosis (AS) [1] . Transapical (TA) TAVR is a substantial risk factor for increased post-procedural mortality [2] . Additionally, decreased Right Ventricular (RV) and Left Ventricular (LV) functions after SAVR or TAVR have been indicated as important risk factors for morbidity and mortality [3,4] . Although, LV and RV systolic and diastolic functional changes after Trans Femoral (TF) TAVR and SAVR have been described in previous studies [5-7] , the influence of moderately invasive procedures, such as TA-TAVR, on LV and RV function has been scarcely investigated, rendering controversial results [8,9] . In AS, the LV responds to chronic pressure overload by developing myocardial hypertrophy, fibrosis, and global systolic and diastolic dysfunctions. Several studies have shown the reversibility of hypertrophy and improvement of systolic function, as well as decreased filling pressures after both SAVR and TAVR [10-15] . In AS with preserved Ejection Fraction (EF), myocardial strain and Peak Systolic Velocity (PSV) by tissue Doppler are highly sensitive markers of LV functional improvement after Aortic Valve Replacement (AVR) [14-17] . In contrast, the Right Ventricular (RV) function and geometry are less affected by severe AS. Thus, despite reduced systolic pulmonary artery pressure, RV function has not been shown to improve, but rather to deteriorate after SAVR. Following open cardiac surgery, both RV geometry and function deteriorate, while the TAVR procedure does not seem to affect RV function [5,7,18] . The present study aimed to investigate late postoperative changes (6-12 months) in LV and RV function after TAVR and SAVR, with a focus on potential differences between TA and TF access for TAVR, compared to SAVR with and without Coronary Artery Bypass Grafting (CABG). We performed conventional systolic and diastolic functional echocardiographic parameters from LV and RV Tissue Doppler Imaging (TDI) and Speckle Tracking Echocardiography (STE) with strain-rate imaging to detect changes after AVR. Since TA-TAVR is more invasive than TF-TAVR, we hypothesized that TA-TAVR might show less LV improvement and decreased RV function. We also investigated whether CABG was an additional factor that improved or deteriorated the LV or RV function.
Study design, setting and population
This single-center study was performed at the University Hospital of North Norway, Tromso. Between February 2010 and June 2013, 175 consecutive patients with severe symptomatic AS eligible for either TAVR or SAVR were included in the study. The indication for TAVR or SAVR was based on a decision made by a multidisciplinary heart team, determined by the patient’s suitability for method, technical feasibility, the risk for open-heart surgery, age, comorbidities, and mental status. Patients with an inability to provide informed consent, a life expectancy of fewer than 12 months, and low motivation for interventional treatment were excluded from the study. All study participants were invited to undergo a preoperative clinical assessment and echocardiography with post-procedural control echocardiography at 6 (range 5-7) and 12 (range 11-13) months. All patients who returned to, at least one of the clinical control visits were included in the study. The study population was divided into four groups; TA- TAVR, TF-TAVR and SAVR with and without CABG. Clinical characteristics, mortality, and perioperative complications were obtained from the patients` electronic journals.
The study was approved by the Regional Ethical Committee of North Norway (REK Nord 397/2010) and all patients provided written informed consent.
Echocardiography
All patients underwent preoperative Transthoracic Echocardiographic (TTE) evaluation using an iE33 scanner (S5-1 probe, Philips Medical Systems, Andover, MA, USA) in the left lateral decubital position. Conventional 2-dimensional greyscale images were obtained in the parasternal long and short axes, as well as in the apical four-, two-, and three-chamber views. The LVEF was derived from the standard biplane Simpson mode. The apical four- and two-chamber views were used to calculate left atrial volumes at end-systole. Mitral Annular Plane Systolic Excursion (MAPSE) and Peak Systolic Velocity (PSV) by tissue Doppler were measured in the septal and lateral mitral rings in the apical four-chamber view, reported as the average of these. Intraventricular septum thickness and LV mass in diastole were measured on M-mode images of the parasternal long-axis view. Diastolic LV function was assessed by E/A ratio, E/e`ratio, E Deceleration Time (DT), E-Wave velocity, the mean of septal and lateral wall tissue Doppler velocities (e´), the systolic filling fraction of the pulmonary veins (PV-SFF), the left atrial volume at end-systole, the peak gradient over the Tricuspid Regurgitation (TRpeak)and the Iso Volumetric Relaxation Time (IVRT). The degree of AS was expressed as the mean gradient estimated from the Doppler flow across the aortic valve and indexed aortic valve opening area, derived by the continuity equation. The LV Stroke Volume (SV), Cardiac Output (CO), and Cardiac Index (CI) were derived from the Left Ventricular Outflow Tract (LVOT) diameter and the LVOT velocity time integral. To assess RV geometry, the longitudinal diameter between the RV apex and center of the tricuspid valve, largest ventricular transverse diameter, RV diastolic area, and RV systolic area were measured in a 4Ch view optimized for the RV ventricle. For RV systolic function, Tricuspid Annular Systolic Excursion (TAPSE), PSV of the free RV lateral wall, and Fractional Area Change (FAC) were measured. RV diastolic function was measured using the tricuspid inflow parameters RV E/A ratio, E Deceleration Time (RV E DT), tricuspid E velocity, tissue Doppler RV Peak Early Diastolic Velocity (RV e´), RV E/e’, and SFF of the Hepatic Veins (HV-SFF). Since a quantitative method for technical reasons was not possible in all patients, we performed a multi-parametric, semi-quantitative evaluation of mitral regurgitation and aortic regurgitation, as recommended in the guidelines [19] .
Strain analyses: Strain and SR analyses were performed using the speckle-tracking software VVI 7 (Siemens, Mountain View, CA, USA). Longitudinal LV strain was obtained by analyzing the LV in the apical four-, two-, and three-chamber views, and circumferential LV strain was obtained by analyzing the mid-ventricular short axis view. Peak systolic strain values were defined as the peak values between aortic valve opening and closure. The timing for strain analysis was derived from Doppler measurements of the aortic and mitral valves. The start of the cardiac cycle was defined as the peak R on the ECG. Regional strain curves with artifacts due to reverberation, air artifacts, missing segments, or insufficient tracking were discarded based on subjective visual assessment. The peak global longitudinal and circumferential strains and Strain Rate (SR) were calculated from a global endocardial curve. In patients with atrial fibrillation, the strain from three cycles, if available, was obtained and then averaged.
The TAVR procedure
All TAVR procedures were performed under general anesthesia with either TF or TA access, using either the self-expanding Medtronic CoreValve (Medtronic Inc., Minneapolis, Minnesota, USA) or the Edwards SAPIEN balloon-expandable valve (Edwards Lifesciences, Irvine, California, USA). TF access was the preferred modality, but TA access was used in the presence of highly calcified and tortious pelvic vessels, given the acceptable LVEF and respiratory function. Other access sites were excluded from this study. The valve was implanted during rapid pacing (180 beats/min). The valve function and degree of valvular leakage were evaluated using TTE before discharge. As this study was performed in the early stage of TAVR, SAVR was performed when the patients were younger than 75 years, the Euro Score indicated low surgical risk, or if CABG was required for sufficient revascularization.
Statistical analysis
All statistical analyses were performed using SPSS 26 (SPSS Inc., Chicago, IL, USA). Since none of the measurements at the 6 months compared to the 12 months follow-up were significantly different, we compared the baseline measurements with the averaged measurements of both follow-up studies using a paired t-test. For patient characteristics and the comparison of pre-to post-procedural findings, one-way ANOVA was performed with Bonferroni post-hoc analysis. Categorical variables were compared using the chi-square test with separate subgroup analyses. P-values<0.05 were regarded as significant.
Reproducibility
To evaluate inter-and intra- observer variability for longitudinal and circumferential strains, 30 patients were randomly selected for repeated strain analysis by the first and second investigators. The variability was calculated as the level of agreement.
Patients
Of the 175 initially included patients, 20 died during the procedure or in the first 6 months following surgery, whereas 8/37(22%), 9/56 (16%), 3/38 (8%), and 0/25 (0%) patients underwent TA-TAVR, TF-TAVR, SAVR, and SAVR+CABG, respectively. An additional 23 patients did not meet either of the follow-up controls, mostly because of the reduced general condition combined with long travel distances across northern Norway. The echocardiographic data of the first visit were lost for one patient, and for one patient, the postoperative imaging results were excluded due to low imaging quality, leaving 130 participants for the final analysis of pre-and at least one of two postoperative clinical and echocardiographic controls in the period from 6 to 12 months. Of the included patients with at least one postoperative control, 21 died between six months and two years after aortic valve replacement. Patient characteristics of the four patient groups are listed in Table 1. The table shows that Coronary Artery Disease (CAD) was present in nearly two-thirds of the patients, while 71/130 (46%) patients were revascularized in connection with AVR investigation or intervention. The patients undergoing TAVR were significantly older and had higher Euro scores. The TA-TAVI group was mainly male, whereas female participants were dominant in the TF group. Only one out of 11 patients with recent myocardial infarction underwent SAVR, and one out of 27 patients with previous CABG was elected for SAVR. As expected, COPD was more frequent in the TAVR group than in the SAVR group. The number of patients with persistent atrial fibrillation or postoperative need for ventricular pacing did not differ significantly between the groups.
| TA-TAVR* | TF-TAVR† | SAVR‡ | SAVR+CABG | All AVR | p-value | |
|---|---|---|---|---|---|---|
| Mean ± SD n (%) |
Mean ± SD n (%) | Mean ± SD n (%) | Mean ± SD n (%) |
Mean ± SD n (%) |
||
| n total | 27 | 43 | 36 | 24 | 130 | |
| Male | 23 (85) | 15 (34)* | 22 (61) | 12 (50) | 72 (55) | <0.001 |
| Age (y) | 83 ± 6 | 83 ± 5 | 77± 5*† | 77± 5*† | 80± 6 | <0.001 |
| BMI (kg/m2) | 26 ± 4 | 27 ± 5 | 28 ± 4 | 27 ± 5 | 27 ± 4 | 0.265 |
| PCI pre TAVR | 12 (44) | 23 (53) | - | - | 35 (27) | 0.756 |
| CAD | 22 (81) | 29 (67) | 11 (31) *† | 24 (100) †‡ | 85 (65) | <0.001 |
| Angina | 21 (78) | 26 (60) | 17 (47) | 16 (67) | 79 (61) | 0.211 |
| Previous CABG | 15 (56) | 11 (26)* | 1 (3) *† | 0 (0)*† | 27 (21) | <0.001 |
| New MI (90 days) | 3 (11) | 7 (16) | 0(0) | 1 (4) | 11 (8) | 0.067 |
| Perifer vascular disease | 11 (41) | 8 (19) | 2 (6)* | 1 (4)* | 22 (17) | <0.001 |
| Cerebrovascular disease | 5 (19) | 10 (23) | 5 (14) | 3 (13) | 23 (29) | 0.549 |
| COPD | 9 (33)* | 9 (26) | 8 (22) | 3 (13) | 33 (25) | 0.275 |
| Cancer | 4 (15) | 8 (19) | 8 (22) | 7 (29) | 27 (20) | 0.598 |
| Hypertension | 16 (59) | 32 (74) | 26 (72) | 17 (71) | 91 (70) | 0.689 |
| Diabetes | 8 (29) | 15 (35) | 7 (19) | 8 (32) | 8 (29) | 0.431 |
| Smoking | 5 (19) | 1 (2) | 3 (8) | 5(21) | 14 (11) | 0.046 |
| Cholesterol (mmol/l) | 4.8 ± 1.5 | 4.9 ± 1.0 | 4.7 ± 1.0 | 4.9 ± 1.2 | 4.8 ± 1.1 | 0.784 |
| GFR pre (ml/min/1.73 m2) | 34 ± 11 | 34 ± 11 | 39 ± 12 | 40 ± 13 | 36 ± 12 | 0.067 |
| GFR post (ml/min/1.73 m2) | 35 ± 12 | 37 ± 13 | 43 ± 18 | 43 ± 16 | 39 ± 15 | 0.065 |
| Heart failure <2 weeks | 20 (74) | 35 (81) | 20 (56) | 16 (67) | 91 (70) | 0.112 |
| CK MB post | 35 ± 101 | 9 ± 4 | 22 ± 9 | 37 ± 31 | 23 ± 49 | 0.056 |
| Atrial fibrillation/flutter preop | 7 (26) | 11 (26) | 6 (17) † | 1(4) † | 25 (19) | <0.001 |
| Atrial ibrillation/flutter postop | 7 (26) | 12 (28) | 6 (17) | 4 (17) | 29 (8) | 0.249 |
| Preoperative ventr pacing or LBBB | 6 (22) | 5 (12) | 3 (8) | 4 (17) | 18 (14) | 0.645 |
| New postoperative ventricular pacing or LBBB | 3 (11) | 3 (7) | 2 (6) | 0 (0) | 8 (6) | 0.507 |
| LogEuroScore | 27 ± 15 | 23± 11 | 9 ± 4*† | 9 ± 6*† | 17 ± 13 | <0.001 |
Note: *: TA-TAVR; †: TF-TAVR; ‡: SAVR. TAVR: Transcatheter Aortic Valve Replacement; TA: Transapical; TF: Transfemoral; SAVR: Surgical Aortic Valve Replacement; CABG: Coronary Artery Bypass Graft; BMI: Body Mass Index; PCI: Percutaneous Coronary Intervention; CAD: Coronary Artery Disease; COPD: Chronic Obstructive Pulmonary Disease; CABG: Coronary Artery Bypass Graft; MI: Myocardial Infarction within 90 days before AVR; GFR: Glomerular Filtration Rate; LBBB: Left Bundle Branch Block, n: Total number of patients.
Table 1: Significance of patient characteristics.01425.
LV systolic and diastolic function: The parameters directly related to a successful AVR were similarly reduced after all procedures, including reduction of the transvalvular gradient, shortening of the ejection time, and reduction of myocardial mass. Table 2 and Figure 1 demonstrate the indicators of systolic and diastolic LV function, overall cardiac performance, and their respective changes in the four groups. The NT-proBNP and NYHA class levels indicated a higher severity of pre-interventional heart failure in TAVR patients, followed by a significant reduction in NT-proBNP and NYHA class improvement in 94% of all patients. Indicators of overall cardiac performance, such as SVI and CI, increased only in the TF-TAVR group. LV volumes, as a marker of general LV function, decreased in all groups except the TA-TAVR group. Longitudinal systolic functional parameters, such as MAPSE, PSV, longitudinal strain, and SR, increased significantly in the TF-TAVR and SAVR groups. However, the TA-TAVR group did not show significant changes in any longitudinal or circumferential functional parameters, except for improved mitral PSV. The circumferential strain and SR increased significantly only after TF-TAVR and SAVR+CABG. In summary, none of the functional parameters indicated a deterioration of LV systolic function after the procedure, while improvement in both longitudinal and circumferential parameters was most marked and consistent in the TF-TAVR group. The diastolic LV parameters are shown in Figure 1 and Table 3. LV E/ e´, TRpeak and IVRT indicated similarly reduced filling pressures after all types of intervention, while SFF of the pulmonary veins decreased and E/A ratio increased after SAVR but remained unchanged after TAVR. Interestingly, a reduction of E/e` was occurring mainly due to increasing e´ at unchanged or even higher E wave velocity. However, differences between the groups regarding pre-to post-procedural changes were not significant, except for the E/A ratio, which increased after SAVR only, indicating higher filling pressures after open surgery. MV e´ (Table 3) increased after TAVR and SAVR, reflecting an improvement of LV relaxation properties.
| Procedure | n | Pre mean ± SD | Post mean ± SD | p-value | |
|---|---|---|---|---|---|
| NT-proBNP (pg/ml) | TA-TAVR | 22 | 4306 ± 7433 | 2001 ± 2561 | 0.117 |
| TF-TAVR | 41 | 5030 ± 8464 | 1856 ± 3389 | 0.004 | |
| SAVR | 35 | 1092 ± 1085 | 761 ± 1064 | 0.094 | |
| SAVR+CABG | 17 | 1151 ± 1479 | 712 ± 787 | 0.078 | |
| NYHA class III-IV (n/%) | TA-TAVR | 27 | 24 (89%) | 1 (4%) | <0.001 |
| TF-TAVR | 41 | 39 (95%) | 3 (7%) | <0.001 | |
| SAVR | 36 | 25 (69%) | 1 (3%) | <0.001 | |
| SAVR+CABG | 24 | 17 (71%) | 1 (4%) | <0.001 | |
| EF Simpson Biplane (%) | TA-TAVR | 27 | 52 ± 13 | 50 ± 9 | 0.39 |
| TF-TAVR | 43 | 52 ± 15 | 55 ± 9 | 0.12 | |
| SAVR | 36 | 56 ± 12 | 60 ± 9 | 0.054 | |
| SAVR+CABG | 24 | 55 ± 9 | 58 ± 9 | 0.179 | |
| LV volume diastole (ml) | TA-TAVR | 27 | 139 ± 60 | 123 ± 32 | 0.181 |
| TF-TAVR | 43 | 121 ± 52 | 101 ± 40 | <0.001 | |
| SAVR | 36 | 121 ± 32 | 99 ± 27 | <0.001 | |
| SAVR+CABG | 24 | 121 ± 42 | 94 ± 28 | <0.001 | |
| PSV mitral ring (cm/s) | TA-TAVR | 27 | 5.3 ± 1.3 | 5.8 ± 1.2 | 0.022 |
| TF-TAVR | 43 | 5.4 ± 1.2 | 6.5 ± 1.4 | <0.001 | |
| SAVR | 34 | 6.5± 1.4 | 7.8 ± 2.8 | 0.011 | |
| SAVR+CABG | 24 | 6.2 ± 1.1 | 6.9 ± 2.5 | 0.089 | |
| Longitudinal Strain ET (%) | TA-TAVR | 26 | -8.2 ± 2.2 | -8.5 ± 2.8 | 0.221 |
| TF-TAVR | 42 | -9.4 ± 2.3 | -9.9 ± 2.6 | <0.001 | |
| SAVR | 34 | -11.0 ± 2.3 | -11.8 ± 2.5 | <0.001 | |
| SAVR+CABG | 24 | -9.3 ± 2.6 | -9.3 ± 3.2 | 0.817 | |
| Circumferential Strain ET (%) | TA-TAVR | 18 | -13.7 ± 3.4 | -14.5 ± 5.0 | 0.257 |
| TF-TAVR | 26 | -13.1 ± 3.5 | -14.1 ± 3.7 | <0.001 | |
| SAVR | 23 | -15.0± 3.2 | -15.3 ± 3.6 | 0.353 | |
| SAVR+CABG | 15 | -14-4 ± 4.5 | -14.5 ± 4.7 | 0.737 | |
| Longitudinal SR ET (1/s) | TA-TAVR | 26 | 0.52 ± 0.12 | 0.53 ± 0.14 | 0.373 |
| TF-TAVR | 42 | -0.57 ± 0.12 | -0.60 ± 0.14 | 0.011 | |
| SAVR | 34 | -0.67 ± 0.11 | -0.71 ± 0.13 | <0.001 | |
| SAVR+CABG | 24 | -0.61 ± 0.12 | -0.64 ± 0.14 | 0.029 | |
| Circumferential SR ET (1/s) | TA-TAVR | 18 | 0.82 ± 0.25 | 0.89 ± 0.41 | 0.179 |
| TF-TAVR | 26 | -0.75 ± 0.19 | -0.84 ± 0.22 | <0.001 | |
| SAVR | 23 | -0.88 ± 0.16 | -0.89 ± 0.22 | 0.570 | |
| SAVR+CABG | 15 | -0.88 ± 0.33 | -0.97 ± 0.40 | 0.036 |
Note: No significantly differing pre-to post AVR changes between groups. TAVR: Transcatheter Aortic Valve Replacement; TA: Transapical; TF: Transfemoral; SAVR: Surgical Aortic Valve Replacement; CABG: Coronary Artery Bypass Graft; NT-proBNP: N-terminal pro-Brain Natriuretic Peptid; NYHA: New York Heart Association; EF: Ejection Fraction; LV: Left Ventricular; PSV: Peak Systolic Velocity; ET: Ejection Time; SR: Strain Rate.
Table 2: Clinical and LV systolic functional parameters by conventional echocardiography and strain rate imaging.
Figure 1: Left ventricular systolic and diastolic functional parameters pre and post aortic valve replacement. Comparison of the different
treatment groups. TAVR, transcatheter aortic valve replacement; SAVR, surgical aortic valve replacement; CABG, coronary artery bypass
graft; MAPSE, mitral annular plane systolic excursion.
Note: ( ) Pre AVR; (
) Pre AVR; ( ) Post AVR; (
) Post AVR; ( ) Pre AVR; (
) Pre AVR; ( ) Post AVR
) Post AVR
| Procedure | n | Pre mean ± SD | Post mean ± SD | p-value | |
|---|---|---|---|---|---|
| E-Wave Velocity (cm/s) | TA-TAVR | 27 | 101 ± 38 | 93 ± 40 | 0.306 |
| TF-TAVR | 43 | 98 ± 26 | 93 ± 30 | 0.399 | |
| SAVR | 36 | 90 ± 22 | 93 ± 25 | 0.452 | |
| SAVR+CABG | 24 | 97 ± 30 | 101 ± 30 | 0.345 | |
| E/A | TA-TAVR | 17 | 1.2 ± 0.7 | 0.8 ± 0.6 | 0.147 |
| TF-TAVR | 29 | 1.0 ± 0.7 | 0.9 ± 0.2 | 0.149 | |
| SAVR | 29 | 0.7 ± 0.2 | 0.9 ± 0.2 | 0.013 | |
| SAVR+CABG | 22 | 0.87 ± 0.24 | 0.93 ± 0.32 | 0.35 | |
| MV e´ (cm/s) | TA-TAVR | 27 | 5.7 ± 1.6 | 5.9 ± 1.2 | 0.552 |
| TF-TAVR | 43 | 5.4± 1.6 | 6.0 ± 1.6 | 0.006 | |
| SAVR | 34 | 6.2 ± 3.8 | 7.2 ± 1.7 | 0.108 | |
| SAVR+CABG | 24 | 5.5 ± 1.6 | 6.9 ± 2.5 | 0.024 | |
| Pulmonary veins: SFF (%) | TA-TAVR | 25 | 42 ± 22 | 44 ± 15 | 0.695 |
| TF-TAVR | 38 | 50 ± 15 | 50 ± 13 | 0.796 | |
| SAVR | 35 | 55 ± 16 | 52 ± 14 | 0.075 | |
| SAVR+CABG | 24 | 59 ± 9 | 52 ± 12 | 0.003 | |
| LA volume index (ml) | TA-TAVR | 27 | 58 ± 20 | 60 ± 39 | 0.797 |
| TF-TAVR | 42 | 55 ± 28 | 51 ± 22 | 0.259 | |
| SAVR | 35 | 44 ± 15 | 42 ± 12 | 0.3 | |
| SAVR+CABG | 24 | 45 ± 16 | 42 ± 15 | 0.236 | |
| IVRT (ms) | TA-TAVR | 27 | 64 ± 30 | 87 ± 35 | 0.036 |
| TF-TAVR | 43 | 71 ± 60 | 88 ± 47 | 0.123 | |
| SAVR | 36 | 72 ± 34 | 82 ± 26 | 0.068 | |
| SAVR+CABG | 24 | 72 ± 38 | 103 ± 46 | 0.024 |
Note: Significant difference towards * TA-TAVR; † TF-TAVR; ‡ SAVR. TAVR: Transcatheter Aortic Valve Replacement; LV: Left Ventricular; TA: Transapical; TF: Transfemoral; SAVR: Surgical Aortic Valve Replacement; CABG: Coronary Artery Bypass Graft; SFF: Systolic Filling Fraction; LA: Left Atrium; IVRT: Isovolumic Relaxation Time.
Table 3: LV diastolic functional parameters before and after different procedures of aortic valve replacement.
RV systolic and diastolic function: The RV geometrical and functional systolic parameters are shown in Figure 2 and Table 4. After SAVR, the RV was shortened and dilated (higher RV area), and both the TAPSE and RV PSV were significantly reduced. RV function after SAVR deteriorated compared to TA-TAVR and TF-TAVR, in which the RV geometrical and systolic functional properties improved. After SAVR, all RV diastolic parameters indicated deterioration of diastolic properties with higher filling pressures (Figure 3), which was significantly different from the unchanged or slightly improved diastolic RV properties after both TAVR procedures. Results of the inter- and intra-observer variability are shown in Table 5. The present study demonstrated that after 6-12 months, LV systolic and diastolic properties improved significantly in all AVR procedures. Thus, the PSV MR, longitudinal strain, and circumferential strain increased significantly after both transcatheter and surgical procedures.
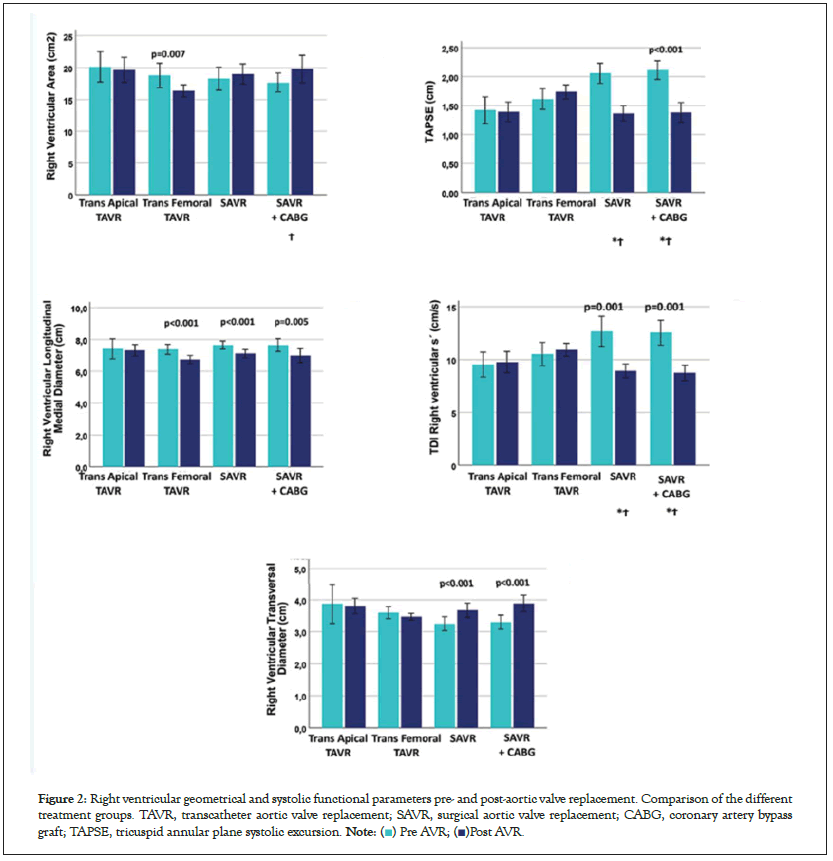
Figure 2: Right ventricular geometrical and systolic functional parameters pre- and post-aortic valve replacement. Comparison of the different
treatment groups. TAVR, transcatheter aortic valve replacement; SAVR, surgical aortic valve replacement; CABG, coronary artery bypass
graft; TAPSE, tricuspid annular plane systolic excursion.
Note: ( ) Pre AVR; (
) Pre AVR; ( )Post AVR.
)Post AVR.
| Procedure | n | Pre mean ± SD | Post mean ± SD | p-value | |
|---|---|---|---|---|---|
| RVOT VTI (cm) | TA-TAVR | 24 | 14.5 ± 3.9 | 15.1 ± 9.3 | 0.776 |
| TF-TAVR | 39 | 14.8 ± 4.6 | 16.4 ± 9.1 | 0.281 | |
| SAVR | 36 | 16.1 ± 6.1 | 16.8 ± 7.2 | 0.653 | |
| SAVR+CABG | 23 | 17.3 ± 10.0 | 14.9 ± 2.8 | 0.248 | |
| RV FAC (%) | TA-TAVR | 27 | 30 ± 14 | 35 ± 9 | 0.033 |
| TF-TAVR | 43 | 32 ± 15 | 33 ± 11 | 0.684 | |
| SAVR | 36 | 34.8 ± 13.7 | 34.1 ± 13.7 | 0.841 | |
| SAVR+CABG | 22 | 35.6 ± 18.0 | 37.8 ± 9.8 | 0.494 | |
| TV E (cm/s) | TA-TAVR | 27 | 45 ± 12 | 48 ± 10 | 0.406 |
| TF-TAVR | 41 | 44 ± 12 | 46 ± 19 | 0.627 | |
| SAVR | 36 | 45.5 ± 9.1 | 48.1 ± 9.4 | 0.158 | |
| SAVR+CABG | 24 | 40.4 ± 9.2 | 51.5 ± 10.5 | <0.001 | |
| TV A (cm/s) | TA-TAVR | 17 | 41 ± 6 | 42 ± 6 | 0.382 |
| TF-TAVR | 27 | 44 ± 9 | 42 ± 11 | 0.267 | |
| SAVR | 29 | 44.0 ± 12.3 | 41.1 ± 7.9 | 0.23 | |
| SAVR+CABG | 22 | 44.2 ± 12.2 | 41.7 ± 9.8 | 0.411 |
Note: No significantly differing pre-to post AVR changes between groups. TAVR: Transcatheter Aortic Valve Replacement; TA: Transapical; TF: Transfemoral; SAVR: Surgical Aortic Valve Replacement; CABG: Coronary Artery Bypass Graft; RVOT VTI: Right Ventricular Outflow Tract Velocity Time Integral; RV FAC: Right Ventricular Fractional Area Change; TV: Tricuspid Valve.
Table 4: RV geometry and functional parameters.
Figure 3: Right ventricular diastolic parameters pre- and post-aortic valve replacement. Comparison of the different treatment groups. TAVR,
transcatheter aortic valve replacement; SAVR, surgical aortic valve replacement; CABG, coronary artery bypass graft; TDI, tissue Doppler imaging.
Note: ( ) Pre AVR; (
) Pre AVR; ( ) Post AVR.
) Post AVR.
| Mean | SD | CI Lower Bound | CI Upper Bound | |
|---|---|---|---|---|
| Intra-observer variability | ||||
| Longitudinal Strain ET (%) | 1.8 | ± 3.0 | -4.1 | 7.7 |
| Circumferential Strain ET (%) | 1.4 | ± 2.8 | -4.1 | 6.9 |
| Longitudinal SR ET (1/s) | 0.08 | ± 0.19 | -0.29 | 0.45 |
| Circumferential SR ET (1/s) | 0.07 | ± 0.21 | -0.34 | 0.48 |
| Inter-observer variability | ||||
| Longitudinal Strain ET (%) | 2 | ± 3.9 | -5.6 | 9.6 |
| Circumferential Strain ET (%) | 2.2 | ± 3.2 | -4.1 | 8.5 |
| Longitudinal SR ET (1/s) | 0.11 | ± 0.21 | -0.3 | 0.52 |
| Circumferential SR ET (1/s) | 0.13 | ± 0.24 | -0.34 | 0.6 |
Note: ET: During Ejection Time; SR: Strain Rate.
Table 5: Bland Altman limits of agreement for myocardial peak systolic strain- and SR measurements.
Diastolic Doppler-based parameters and NT-proBNP levels indicated a reduction in LV filling pressures, which coincided with substantially improved symptoms. However, following surgical procedures such as SAVR and SAVR with CABG, RV systolic and diastolic function worsened, while TA-TAVR and TF-TAVR showed similarly unchanged indicators of RV systolic and diastolic function. There were no significant differences in pre-to post- procedural changes between SAVR with and without CABG.
LV systolic function: In this non-randomized study, patients were consecutively included as they were assigned to different procedures. Therefore, the TA and TF-TAVR groups were significantly older and had lower preoperative LV systolic deformations. NT-proBNP and diastolic parameters indicated higher pre-procedural filling pressures in the TAVR group than in the SAVR group. However, the long-term effect of afterload reduction similarly improved the long- and short-axis deformations after all procedures. This was reflected by the post-procedural improvement in longitudinal strain, SR, PSV, and circumferential strain. In contrast to strain and SR, EF was less sensitive to significant changes in any of the procedures, but the mean values (except for TA-TAVR) indicated a tendency towards improvement. Changes in LV function after SAVR and TAVR have been the focus of several studies [17, 20- 26] . A review by Garg et al. in 2017 summarized these changes with uniformly increasing longitudinal and circumferential strain, regional function, and twist, whereas EF was reported as either higher or unchanged [22] . TA access for TAVR has emerged as a significant risk factor for early post-procedural mortality [2,27,28] . The following question is whether the increased mortality in TA- TAVR could be partly due to the changes in the left ventricular functional properties. Previous echocardiographic and cardiac magnetic resonance studies have indicated reduced regional apical, radial, and longitudinal strains, and in some studies, neither global strain nor EF improved after TA-TAVR [24,29] . These findings were confirmed in the present study, in which TA-TAVR was the only procedure without significant improvement in longitudinal or circumferential strain and SR. However, none of these parameters showed deterioration of LV function. As untreated AS comprises severe systolic and diastolic dysfunction, improvement in LV function seems to be an important factor for positive outcomes, and a lack of LV functional improvement might be connected with a higher risk of TA-TAVR.
LV diastolic function: As previously described in the same AS population [30] , diastolic dysfunction consists ofboth impaired relaxation and increased end-diastolic pressure due to increased ventricular and atrial filling and myocardial stiffness following fibrosis. Signs of increased stiffness due to high filling pressures are expected to reverse as a result of afterload reduction [31] , whereas stiff myocardium due to macro- or micro-scarring is expected to render irreversibly high filling pressures [32] . Accordingly, successful AVR led to signs of decreased filling pressures; thus, peak pulmonary artery pressure and MV E/e´ decreased, and IVRT increased similarly after all types of procedures. However, in contrast to TAVR, after SAVR, the E/A ratio increased, and the SFF of the pulmonary veins decreased significantly. One explanation might be that E and A wave velocities and pulmonary venous flow are more dependent on ventricular elasticity rather than filling pressure. Based on this observation, we hypothesize that surgical procedures may lead to micro-scarring, especially after combined procedures, while LV elasticity after TAVR, even with TA access and remains unchanged. Impaired relaxation seems to be reversible to some degree when stenosis is removed and ventricular pressure decreases, while age-related reduced relaxation remains. The best indicator reflecting improved relaxation was e’, which increased after all types of procedures, while DT, as a second relaxation indicator, improved significantly only after TF-TAVR. For TA-TAVR, only IVRT was significantly changed, but the small group size left the measured effect sizes generally underpowered. However, interpreting trends and effect size, the diastolic properties before and after intervention were similar in both TAVR groups, with substantially reduced filling pressures and improvement of early relaxation, while elasticity or compliance of the LV seemed to be unchanged.
RV systolic and diastolic function: In contrast to the improvement of LV function, RV systolic function is consistently reported to be reduced after SAVR, but improved or unchanged following TAVR [5,7] . In our study, the RV longitudinal diameter decreased in both SAVR and TAVR; as previously described, the transverse diameters increased only after SAVR. Okada et al. reported a reduction in these diameters after both TA-TAVR and TF-TAVR, whereas our data showed a slight reduction in both diameters and the RV area only in the TF-TAVR group, while the area after TA-TAVR remained unchanged. Reduction in the RV area can be regarded as a sign of overall improved RV function. Open surgery was followed by a reduction in RV systolic function, while RV systolic parameters remained unchanged after TAVR. The reduction in TAPSE and RV PSV after open surgery is a known phenomenon and appears also to be present in minimally invasive procedures [33] . MR studies have reported reduced RV EF and new RV scars after SAVR [34,35] . In addition to decreasing systolic RV function, in the present study, we observed a decrease in RV diastolic function after SAVR. Increasing E/e’ and decreasing DT and SFF of the hepatic veins indicated increased RV filling pressures and/or loss of RV elasticity. Decreasing e’ of the RV lateral wall resulted in reduced relaxation properties. Our findings on systolic and diastolic RV function support previous observations of the negative impact of SAVR, in which the most plausible cause seems to be micro- scarring due to cardioplegia and extra-corporal circulation [34] . TA- TAVR with a small pericardial incision does not interfere with RV structures and does not seem to affect RV configuration and function.
Overall performance and clinical improvement: The overall cardiac performance indicated by CI and SVi increased only significantly after TF-TAVR, while it remained unchanged after the other procedures. Since LV function improved after SAVR, the unchanged overall cardiac performance could be related to reduce RV function. In TA-TAVR, RV function remained unchanged, while the improvement in LV systolic and diastolic function was less pronounced. TA access is an independent risk factor for early post-procedural mortality [2] . Thus, our data reflect a bias towards survivors. The early post-procedural function was not investigated in this study, but it has been previously shown to impact outcomes [3,4] . The subjective reported improvement after AVR was convincing: 81% of 130 participants presented pre-procedural NYHA III-IV versus 5% of patients post-AVR. The NT-proBNP level, an indicator of diastolic filling pressure, decreased in all the groups. Even though NT-proBNP levels were higher in the pre-operative TAVR patients, not just due to age, the reduction of NT-proBNP was most pronounced in the TF-TAVR patients. Our data indicate that this minimally invasive procedure results in the best overall cardiac performance after 6-12 months, whereas the reversibility of heart failure symptoms seems to be more dependent on successful afterload reduction. However, postoperative improvements in RV and LV function and reversibility of NT-proBNP after TAVR and SAVR have an impact on outcome and exercise capacity [4,36] .
Six to twelve months after AVR, heart failure symptoms were reduced in most of the patients, with no difference between treatment groups, LV systolic function and indicators of LV filling pressures improved after all types of intervention. However, we found significant differences in several post-procedural LV and RV functional parameters depending on the type of intervention. The most significant improvement in CI after TF-TAVR indicated the best overall cardiac performance after the least-invasive procedure.
This study has several limitations. First, owing to the high mortality in the TA-TAVR group, only 28 of the initially 37 included patients were available for follow-up examinations. This left our analyses underpowered due to small effect sizes. However, 24 and 28 patients in the small group were sufficient to show clinically relevant large effect sizes. Smaller changes, without statistical significance, could indicate the direction of change or differences between the SAVR and SAVR+CABG groups. Second, the study does not reflect the TAVR population to date, as it was conducted between 2010 and 2013 when the first regular TAVR at our hospital started in 2008. The patient population undergoing TAVR has profoundly changed, and TA-TAVR has become a rare procedure. Thus, a similar study on TA-TAVR with sufficient patient numbers would be difficult to perform to date. Third, this study was not randomized; leading to intervention groups comprising different age groups with differing symptoms, cardiac functions, and risk profiles. In the elderly age group with more severe AS, the LV and RV functions were significantly poorer, and the post-procedural functional recovery might have been impaired due to this. However, our data aimed to show clinically relevant post-procedural changes in comparison to the preoperative state. The advantage of less invasive procedures could be demonstrated since TF-TAVR showed the most significant improvement in overall cardiac performance even in a group with higher age and more myocardial scarring.
Ethics approval and consent to participate
The study was approved by the Regional Ethical Committee of North Norway (REK Nord 397/2010). Written informed consent was obtained from the patients for publication of their individual details and accompanying images in this manuscript. The consent form is held by the author’s institution and is available for review by the Editor-in-Chief.
The data are stored as a research file at the University Hospital in North Norway with limited access to dedicated researchers.
The authors declare that they have no competing interests.
This work was funded and supported by the Regional Health Authorities of North Norway (HN-ID 6884/SFP1078-12) and (HN SFP 1172-14).
AR conceived, designed the study, handled the functioning, acquired data, performed statistical analysis and wrote the manuscript. SM acquired data. DK performed strain-analysis. AR performed statistical analysis. HS and SM made critical revision of the manuscript for important intellectual content.
We would like to thank Editage (www.editage.com) for English language editing.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar][PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar ][PubMed]
[Crossref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
Citation: Rosner A, Kjonass D, Malm S, Schirmer H (2023) Left and Right Ventricular Functional Changes after Transapical, Transfemoral, Transcatheter and Surgical Aortic Valve Replacement: A Single-Center Observational Study. J Clin Exp Cardiolog.13:762.
Received: 21-Dec-2022, Manuscript No. JCEC-22-21103; Editor assigned: 23-Dec-2022, Pre QC No. JCEC-22-21103 (PQ); Reviewed: 06-Jan-2023, QC No. JCEC-22-21103; Revised: 17-Jan-2023, Manuscript No. JCEC-22-21103 (R); Published: 24-Jan-2023 , DOI: 10.35248/2155-9880.23.13.762
Copyright: ©2023 Rosner A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.