Medicinal & Aromatic Plants
Open Access
ISSN: 2167-0412
ISSN: 2167-0412
Review Article - (2020)Volume 9, Issue 3
Cnidoscolus tehuacanensis is an endemic medicinal species used to treat some inflammatory diseases, and biosynthesizes triterpenes and sterols, being Lupeol Acetate (LuAc) the main compound. It was submitted to biological assay to determine the anti-inflammatory and leishmanicidal potential.
Anti-inflammatory effect was determined on acute (12-O-tetradecanoylphorbol-13-acetate -TPA- and Carragenan models) and chronic inflammatory models (induce with Complet Freud´s Adjuvant -CFA-) using Indomethacin and Phenylbutazone (PBZ) as reference drugs. In vitro leishmanicidal activity was determined on Leishmania mexicana promastigotes and amastigotes, Miltefosine and amphotericin B were used as reference drugs.
In TPA assay, LuAc showed an ED50=1.79 mg/ear (Ind, ED50=0.91 mg/ear) and on Carragenan model, the antiinflammatory effect was not dose-dependent, in this case LuAc at 100, 150 and 250 mg/kg showed 43.31, 40.22 and 51.25% of inhibition, respectively at 5 h. On CFA chronic inflammatory model, LuAc and PBZ groups showed similar anti-inflammatory effect and similar Body Weight (BW) gain although this value was less respect to healthy and CFA groups. Also, inhibited the COX-2 activity being this effect slightly better that PBZ, and the joints and soft tissue from LuAc and PBZ groups did not show any alteration with respect to CFA group. In soft tissue, CFA group showed a significant increase size and leukocyte infiltrate as a result of the chronic inflammatory process; it group also showed sinusoidal hyperplasia in the joint. In liver histological analysis, the healthy and LuAc groups showed no alteration, and the CFA group showed a slight microabscess and scarce hematopoiesis, while the PBZ group presented scarce steatosis. The fraction (rich in LuAc) and LuAc pure were less active that extract against L. mexicana promastigotes.
C. tehuacanesis is a potential source of LuAc. This natural compound showed a good anti-inflammatory activity (similar effect to PBZ) and did not cause liver damage.
Lupeol acetate; Chronic anti-inflammatory activity; CFA-model; Leishmanicidal activity; Leishmania spp
Lupeol acetate (LuAc) is the main compound isolated from aerial parts of the Cnidoscolus tehuacanensis Breckon (C. tehuacanensis); it is an endemic species located in the Biosphere Reserve located within the valley of Tehuacán, Puebla and Cuicatlán, Oaxaca and is commonly known as "mala mujer or bad woman". In Calipán, Puebla, the aerial parts of the plant are used to treat problems related to inflammation and as an antiseptic for the treatment of lesions and skin infections [1,2]. Other minor compounds reported from aerial parts of this medicinal plants are β-amyrin acetate, lupeol, lupenone, α-and β-amyrin, amyrenone, germanicol (3β-olean-18-en- 3-ol), β-betulin (lup-20(29)-ene-3β,28-diol), 9,19-cyclolanost-24-en-3- ol acetate, lanosterol acetate, isoorientin and amentoflavone [2]. It has been reported that LuAc suppresses the expression of cytokines related with inflammation, avoids bone erosion and improves symptoms in cases of Rheumatoid Arthritis (RA); in addition, it favored osteoclastogenesis in in vitro model using the RAW 264.7 cell line. In this study, LuAc (80 μM) and liposomal formulation of LuAc at 40 and 80 μM, and curcumin (reference drug, 10 μM) was assayed [3]. An in vivo RA model that employed DBA/1J male mice was found that LuAc showed a good anti-arthritic activity, because it decreases the inflammatory process [3]. In another study, was reported that LuAc (administered every 48 h by oral via, from day 32 to 40, post adjuvant administration) at 66 mg/kg shown anti- AR effect in a model of Complet Freud´s Adjuvant (CFA)-induced arthritic rats [4].
It is well known that the natural products isolated from medicinal plants are a good source of anti-inflammatory compounds such as apigenin, berberine, quercetin, resveratrol, α-humelene and others [5,6]. In particular, the triterpenes have wide structural diversity due to modifications of base skeletons such as oleanane, ursane, and lupane; these three nuclei are the most important, where more than 50,000 isolated triterpenes of different plants have been reported. In addition, these compounds have several biological activities, such as antimicrobial, anti-cancer, antitumor, hypoglycemic, hypolipidemic, anti-malaric, hepatoprotective, cardioprotective, anti-arthritic, analgesic, and anti-inflammatory effects in acute and chronic inflammatory conditions such as chronic obstructive pulmonary disease, osteoarthritis (OA), RA, chronic fatigue, multiple sclerosis, neurological diseases (Alzheimer and Parkinson), cancer, atherosclerosis and other diseases (type 2 diabetes mellitus, obesity, stroke) [4,7-16].
On the other hand, is well known that the chronic diseases appear and increase very frequently with age, coupled with lifestyles (due to alcohol consumption, tobacco, stress, lack of exercise and excessive consumption of red meat and/or fatty foods). For example, in USA, people over 65 years old have at least one chronic condition (80%) and 50% have two or more chronic conditions [10]; in addition, it is the main cause of death (70% of all deaths) or disability in that country. It is well known that in adulthood appear chronic inflammation that generates many Free Radicals (FR) and that FR damage cells, increase or alter signaling molecules (such as cytokines) and transcription factors in several chronic diseases, as well as accelerating their progression or persistence. Among the chronic degenerative diseases is RA or osteoarthritis, which is an autoimmune disorder with high levels of cytokines (CRP, TNF-α, IL-6, IL-1β and IL-8) that cause or contribute to persistent inflammation and joint wear. This sickness (RA) has a profound economic, personal and social impact, whose cost of treatment and/or disability exceeds 1% of gross domestic product in the US [17]. RA is a disease closely related with excessive activation of macrophages and their release of cytokines due to macrophage activation and attraction of more immune cells to infiltrate and provoke the severe inflammatory response.
Epidemiological data show that in the USA every second a patient is diagnosed with a chronic inflammatory condition and 60 million Americans suffer from multiple chronic inflammatory diseases at present, and RA and OA cases are the most frequent cause of physical disability among people over 40 years old around the world. It is predicted that by 2030, approximately 20% of adults will have developed some type of arthritis in Western Europe and North America [15,18]. Due to this, it is necessary to continue carrying out the biological evaluation of compounds that allow the development of new anti-inflammatory agents or innovation in new therapies that allow to be combined with the current ones.
On the other hand, leishmaniasis (caused by about 20 Leishmania spp) is a neglected tropical disease which remains a major global health problem, because the therapeutic options are limited, although it has significant adverse effects (kidney failure, acute pancreatitis, cardiotoxicity, peripheral neuropathy, hepatotoxicity) and emergence of drug resistance is a further complication; it is endemic in 98 developing countries. Today, co-infection of leishamiasis with HIV/SIDA has a poor prognosis, and the treatment is very complicated and long. In addition, some drugs are expensive and some of them are scarcely effective due to drug resistance [19,20]. In this manuscript, we describe the isolation of LuAc from C. tehuacanensis and the anti-inflammatory activity in experimental CFA chronic inflammatory model, as well as the leishmanicidal effect of the LuAc and C. tehuacanensis hexanic extract. Also, we describe the additional compounds detected in this extract.
General experimental procedures
Melting points (m.p.) were determined using a Fisher-Johns apparatus and are uncorrected. Open Column Chromatography on Normal phase (CC-NP) was carried out on silica gel 60 (70-230 mesh; Merck, Darmstadt, Germany). Thin-Layer Chromatography (TLC) analyses were performed on silica gel 60 F254 plates (Merck) and spots were visualized by spraying with a 10% solution of aqueous H2SO4 followed by heating to 100°C to identify LuAc. LuAc was identified by X-ray and Rt in GC-MS analyses.
Mass Spectra (GC-MS) in Agilent (Santa Clara, CA, USA) 6890 N Gas Chromatograph (GC) with an Agilent 7683B automatic liquid sampler was coupled to a LECO Pegasus 4D mass spectrometer. The GC furnace initial temperature (temp) was maintained at 40°C for 3 min, then increased to 300°C for 20°C/min and held for 5 min. The injector temperature was maintained at 300°C using splitless injection mode (2 min). Helium was used as carrier gas with a constant flow-rate of 1 mL/min. The MS was operated in scan mode from 45-500 m/z; ion source temp was set at 200°C; ionization was performed in the impact ionization mode (I.I.) with ionization voltage set at 70 eV. Lipophilic compounds were identified by comparing their MS with those reported in the Publish/National Institute of Standards (NIST) MS Library.
Run conditions for terpenes in GC-MS: Injector temp 280°C; injection mode: split; radius: 80:1; split flow: 55.4 mL/min; running flow: 37 cm/sec; injection volume: 2 μL. Furnace: Initial temp at 85°C; heating ramp: 50°C/min at 290°C,second ramp: 2°C/min to 300°C, held for 19.40 min; total running time: 30 min; transfer line: 290°C.
Chemicals and Standards substances for the anti-inflammatory activity assays, 12-ortho-tetradecanoylphorbol-13-acetate (TPA, Sigma), Carrageenan λ (Sigma), Complete Freund's Adjuvant (CFA, DIFCO), Indomethacin (Sigma) and Phenylbutazone (PBZ, Sigma) were used; Tween 80 (Sigma) or acetone (JT Baker) was used as vehicle and measurement of subplantar edema was performed with a digital micrometer (Mitutoyo, APB-2D).
The HPLC conditions used by analysis of the extract and compound has been described previously [21] with some modification on flow (isopropanol:acetonitrile 1:24 with flow 0.9 mL/min, from 0 to 10 min); 0.7 mL/min, from10-15 min and 0.9 mL/min, 15-20 min).
Plant material, Hex extract preparation, and chemical fractionation
LuAc was obtained as white crystals from hexanic extract of the Cnidoscolus tehuacanensis (leaves). Plant material was collected in Calipán, Coxcatlán, Puebla in October, 2017, and a voucher specimen was deposited under reference number 16,306 in Herbarium IMSSM. The hexanic extract was prepared following the procedure previously described [2] and LuAc was obtained after several CC-NP on silica gel; it shows a Rt=26.15 min in GCMS chromatogram. Briefly, 150 g of dried leaves was extracted by exhaustive maceration at room temperature with hexane 100%, yielding 6 g (4.13%) of the crude extract (Cteh).
The Cteh extract (6 g) was further subjected to CC-NP over silica gel and eluted with Hex 100% (1-71), Hex:CHCl3 9:1 (72- 79), Hex:CHCl3 8:2 (80-83), Hex:CHCl3 6:4 (84-92), Hex:CHCl3 4:6 (93-100), Hex:CHCl3 2:8 (101-117), CHCl3 100% (118-126), affording 56 fractions of 250 mL each. According to the TLC profile, these were combined into 8 groups. Primary fraction 40-58 (1,380 mg, eluted with Hex 100%) was analyzed by GC-MS and was submitted to next CC-NP, because it yields a LuAc with a higher degree of purity. This last CC-NP was eluted with Hex 100% and 55 secondary fractions were obtained. From fraction 40-55 (984 mg), a white crystal was obtained with m.p. 208-210°C (215-218°C, reported Musayeib et al. [22], this sample was submitted to GC-MS, H1-NMR, X-Ray analyses and biological assay.
Leishmanicidal activity
The Cteh extract, primary fraction and pure compounds was assayed against L. mexicana (MNYC/BZ/62/M379) promastigotes and amastigotes, employing the assay previously described [20]. Miltefosine (Milt) and Amphotericin B (AmpB) were used as reference drugs (100 to 0.8 mg/mL); all assays were carried out in triplicate. Also, the half cytotoxicity concentration (CC50) of sample was determined using murine macrophage cell line J774.2 (ATCC TIB-67), and selectivity index (SI) was calculated using the values of CC50/IC50. The sample was dissolved in DMSO and diluted in culture medium at concentration range from 100 to 0.8 mg/mL in a final volume of 200 μg/mL.
In vivo biological assays
Adult Balb/C male mice (25 ± 3 g) were obtained from Animal Vivarium of National Medical Center XXI Century, Mexican Social Security Institute, Mexico City, for the evaluation of antiinflammatory activity, and were maintained under standard laboratory conditions (25°C, 12-h dark/12-h light, 50% relative humidity) according to the Norma Official Mexicana (Mexican Official Norm) NOM-062-ZOO-1999. Food and water were provided ad libitum. Project was approved by National Commission of Scientific Investigation and Bioethics with code CNIC-IMSS R-2018-3601-056.
Acute topical and systemic anti-inflammatory activities
These tests were performed as previously described [2,23].
Chronic inflammatory model induced with CFA
This experiment was carried out according to previously described [24,25], with modifications. 20 μL of CFA was injected subcutaneously in the right hind paw on days zero and 14 day (re-injection). PBZ (100 mg/kg), and LuAc (125 mg/kg) were administered by i.g. route daily from day 7 to 28, the samples (PBZ and LuAc) were solubilized in vehicle [Tween 80: water (1:9)]. Control group and chronic inflammation group received only vehicle. Paw edema was measured at different times (days 1, 4, 7, 11, 13, 15, 17, 19, 21, 25 and 28) (Et) with a digital micrometer (Mitutoyo) and the value of day zero (Eo) was determined as baseline. Body Weight (BW) gain was also registered on the same days compared to day zero. The inhibition percentage of edema development in each group was calculated from day 15 to 28 by comparison with CFA un-treated group, using the following formula:
%Inhibition= (Et-Eo)CFA group – (Et-Eo)treated group/(Et-Eo) CFA group × 100
Lymphoid cell quantification by cell Surface staining and flow cytometry
After 28 d treatment, a pool of popliteal lymphoid node of the right hind paw was obtained for each group. The lymphoid nodes were disrupted and filtered in cold ISS and were placed in RPMI medium with 10% FBS. The cell suspension was incubated with fluorescently labelled antibodies (LIVE/DEAD Fixable Aqua Dead Cell (Invitrogen, US) APC-Vio770 anti-mouse CD3-REA641clone, PE-Vio770 anti-mouse CD4-3 GK1.5 clone (Miltenyi Biotec, Germany)), for 20 minutes at 4°C in staining buffer (PBS with 0.5% BSA and 0.01% sodium azide). The cells were then washed and fixed in 2% paraformaldehyde (Sigma Aldrich, St Louis MO, USA). The data were acquired using a MACS flow cytometer analyzer (Miltenyi Biotec, Germany) and analyzed with FlowJo software (Tree Star, Ashland, OR, USA) [24,26]. Lymphoid cells were gated for analysis of CD3+, CD4+ and CD8+ T cells.
Histological analysis
The popliteal lymphoid node, right hind paw, paw edema, and liver of the male mice were removed and tissue biopsies were fixed in 10% formalin and then embedded in paraffin; these blocks were cut into slices of 4 to 5 m with a rotary microtome (Thermo Scientific, Microm HM 340E) and stained with hematoxylin and eosin (H&E). The stained slices were examined under a light microscope and compared with control group.
Cyclooxygenase (COX-2) inhibition assay
The effect of LuAc on COX-2 mediated PGE2 production was measured using the Chemiluminescent COX (ovine) Inhibitor Screening Assay kit from Cayman Chemical (700200). COX activity was determined by measuring the synthesis of PGE2 according to the instructions provided with the kit. A standard curve with COX-2 (0, 0.008, 0.016, 0.030, 0.063, 0.125, 0.250,0.500 U/ μL) was generated at the same time and from the same plate and was used to quantify PGE2 levels produced in the presence of popliteal lymphoid node of control and treated groups [27]. The concentration of COX-2 present in the groups was calculated from a linear regression (y=mx+b) obtained from the standard curve previously performed with COX-2. The popliteal lymphoid node was placed in phosphate buffer (PBS, pH=4) and the tissue were homogenized in 500 μL of TRIS-HCL buffer (pH=7.5, as proteases inhibitor) and the homogenate was centrifuged at 10000 rpm/15 min/4°C; after that, the supernatant was stored at -80°C until use.
Statistical analysis
Sigma Plot ver. 12.5 statistical software was utilized for analysis of results and graphic elaboration. Data is presented as Standard Error of the Mean (SEM). Significance for the inflammation experiments was determined by one-way analysis of variance (ANOVA) followed by post hoc Student Newman-Keuls. The TPA results was analyzed a one-way ANOVA, while BW gain and development of paw edema for systemic inflammation (Carrageenan model) and Chronic inflammation (CFA) were analyzed with ANOVA and with a post hoc Student-Newman-Keuls (SNK) test; p <0.05 was considered statistically significant. Finally, for CD3+, CD4+ y CD8+ cells percentage values, a Kruskal-Wallis test (ANOVA by Ranks) was carried out, in addition to a post hoc SNK test, in which relevant outcomes were those with a value of p <0.05.
Lupeol acetate obtention from hexanic extract of the C. tehuacanensis
From dry and ground material (150 g), 6.19 g of Cteh extract was obtained, with a yield of 4.13% with respect to material dry weight. Cteh extract (6 g) was submitted to two successive CC-NP. In primary fraction 40-58 (1400 mg, eluted with Hex 100%), LuAc was detected as a main compound, it was obtained as white powder with m.p. 203-205°C and Retention factor (Rf)=0.55 in TLC, using Hex:EtOAc (95:5) as elution system and sprayed with H2SO4 10%; in addition, this primary fraction was analyzed by GC-MS (Figure 1). In chromatogram, a main peak was observed with Retention time (Rt) = 25.10 min. This fraction (1.380 g) was submitted to the next CC-NP. In this case, the silica gel (55.2 g) was impregnated with 25 mL of AgNO3 10% and was eluted with Hex 100%, from this last column 55 secondary fractions (250 mL each) were obtained. From secondary fraction 40-55, 984 mg of white crystals was obtained with m.p. 208-210°C (215-218°C, reported by [22]), soluble in Hex or CHCl3 with Rf=0.55 in Hex:EtOAc (95:5). The GC-MS chromatogram showed a main compound with Rt=25.16 min (Figure 2). The chemical structure of LuAc was corroborated with X-ray (Figure 3). The LuAc isolated from Cteh was used as reference standard by HPLC analysis (Rt=11.39 min, flow 0.9 mL/ min) of the cell suspension culture from C. chayamansa organic extract [21]. The HPLC chromatogram from Cteh also showed the presence of α- and β-amyrine acetate (104.08 and 11.53 mg/g of dry Cteh extract) with Rt=11.34 and 11.89 min; also, LuAc was detected at Rt=14.16 min. In this case, the elution system was the same as previously described [21], (isopropanol:acetonitrile 1:24) with flow 0.9 mL/min (from 0 to 10 min), 0.7 mL/min (from 10-15 min) and 0.9 mL/min (from 15-20 min) (Figure 4).
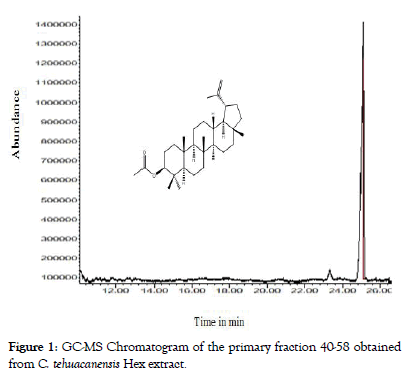
Figure 1: GC-MS Chromatogram of the primary fraction 40-58 obtained from C. tehuacanensis Hex extract.
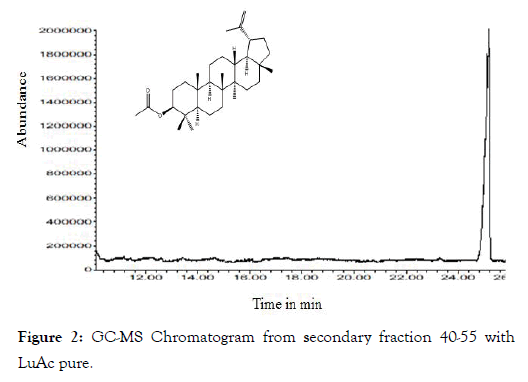
Figure 2: GC-MS Chromatogram from secondary fraction 40-55 with LuAc pure.
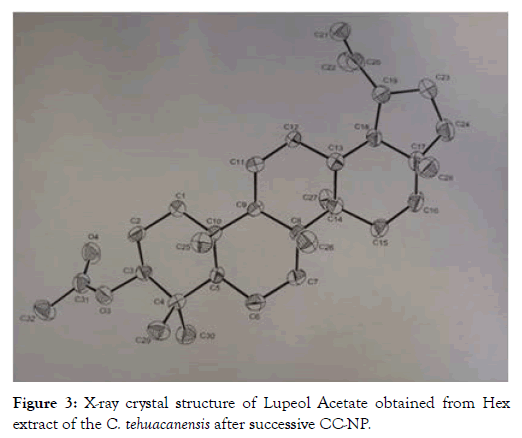
Figure 3: X-ray crystal structure of Lupeol Acetate obtained from Hex extract of the C. tehuacanensis after successive CC-NP.
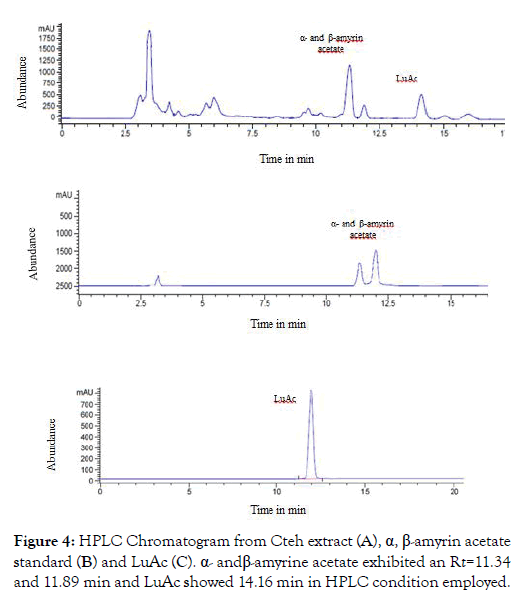
Figure 4: HPLC Chromatogram from Cteh extract (A), α, β-amyrin acetate standard (B) and LuAc (C). α- and β-amyrine acetate exhibited an Rt=11.34 and 11.89 min and LuAc showed 14.16 min in HPLC condition employed.
Leishmanicidal activity
The Cteh extract showed an IC50=58.98 and 8.73 μg/mL against L. mexicana promastigotes and amastigotes, respectively, with a selectivity index (SI)=2.11 and 14.31, by promastigotes and amastigotes. In addition, only the primary fraction (rich in LuAc) and pure LuAc were active, showing IC50=95.29 and 82.02 μg/ mL against L. mexicana promastigotes, and IC50=75.21 and 52.33 μg/mL for amastigotes, respectively. In this case, Milt showed an IC50=15.34 and 0.51 μM and SI=10.23 and 307.8 against L. mexicana promastigotes and amastigotes, respectively. AmpB showed an IC50=0.95 and 0.87 μM and SI=6.5 and 7.11, respectively, against L. mexicana promastigotes and amastigotes. Leishmanial infection is considered a neglected disease without effective treatment and it is urgent to find new molecules with leishmanicidal activity, because the current chemotherapy available is far from satisfactory and has several adverse side effects [19,20]. This is the first manuscript to describe the leishmanicidal activity for LuAc. Previously, it has been described that lupeol exhibited leishmanicidal activity against promastigotes and amastigotes of L. donovani with IC50=65 and 15 μg/mL, respectively [28]. In this case, LuAc showed an IC50=82.02 μg/mL against L. mexicana promastigotes, being this value slightly lower than that reported for lupeol against L. donovani promastigotes (IC50 = 65 μg/mL). In contrast, the IC50 value (52.33 μg/mL) against L. mexicana amastigotes was lower than that reported for lupeol (IC50=15 μg/mL) against L. donovani amastigotes. It is necessary to perform the evaluation of LuAc in an animal model to determine its leishmanicidal potential.
Anti-inflammatory activity by topical (TPA) and systemic (carrageenan) models
The topical anti-inflammatory results are shown in Table 1. LuAc showed an ED50=1.76 mg/ear, value similar to reference drug (Ind, ED50=0.91 mg/ear). The anti-inflammatory results for LuAc on carrageenan model are described in Table 2. In this assay, the LuAc was tested at 20, 50, and 100 mg/kg in a first experiment and in second experiment, it was tested at 150 and 250 mg/kg. The results at 5 h showed that LuAc has a good activity, in this case the inhibition was 33.05, 33.62 and 43.3% at 20, 50 and 100 mg/ kg, respectively; and the inhibition was 40.22 and 51.25 % at 150 and 250 mg/kg. These results indicate that the effect for LuAc is not dose-dependent. Although, the inhibition % was better than Ind (10 mg/kg, reaching 31.33%), the anti-inflammatory effect was lightly maintained until 7 h.
| Treatment | Auricular Edema (mg) | Inhibition Percentage | ED50 (mg/ear) |
|---|---|---|---|
| TPA | 7 ± 0.20bcde | -- | -- |
| Ind (2 mg/ear) | 3.04 ± 0.52a | 56.57 | 0.91 |
| LuAc (0.5 mg/ear) | 3.86 ± 0.72a | 45.63 | 1.76 |
| LuAc (1 mg/ear) | 3.2 ± 0.35a | 54.93 | |
| LuAc (2 mg/ear) | 1.92 ± 0.27a | 73.69 |
Table 1: Anti-inflammatory activity of LuAc on the TPA model.
| Treatment | Paw edema formation (mm) and inhibition percentage | |||
|---|---|---|---|---|
| (mg/kg) | 5 h | 7 h | ||
| mm | Inhibition % | mm | Inhibition % | |
| Carrageenan | 0.8 ± 0.17bcdefg | 0.64 ± 0.1bcdefg | ||
| Ind (10) | 0.55 ± 0.05a | 31.33 | 0.67 ± 0.03a | 13.9 |
| LuAc (20) | 0.54 ± 0.03a | 33.05 | 0.58 ± 0.03a | 9.9 |
| LuAc (50) | 0.53 ± 0.03a | 33.62 | 0.48 ± 0.05a | 25.2 |
| LuAc (100) | 0.46 ± 0.03a | 43.31 | 0.40 ± 0.04ab | 37.5 |
| LuAc (150) | 0.63 ± 0.06a | 40.22 | 0.55 ± 0.05a | 37.7 |
| LuAc (250) | 0.51 ± 0.06a | 51.25 | 0.53 ± 0.02a | 40.01 |
Table 2: Anti-inflammatory activity of the LuAc on the subplantar edema induced with carrageenan.
Previously, we described that LuAc is one of the main compounds and it is responsible for the topical and systemic anti-inflammatory activity shown by the CHCl3:MeOH extract from C. tehuacanensis (leaves), an endemic medicinal plant located inside the Tehuacán- Cuicatlán biosphere reserve, Mexico [2]. On the other hand, it has been described that LuAc showed in vitro anti-inflammatory activity in two cell line (RAW 264.7 cell and Bone marrow-derived macrophages -BMDMs- stimulated with lipopolysaccharide) [9]. In addition, Lucetti et al. [29] described that LuAc (isolated from Himatanthus drasticus) showed analgesic (formalin assay) and antiinflammatory activities (in carrageenan and dextran assays) using male Swiss mice. The anti-inflammatory effect of LuAc (50 mg/ kg) was better than Ind (10 mg/kg in paw edema induced with carrageenan at the 3rd h). On the other hand, in dextran model, LuAc at 1.2 and 25 mg/kg (administered by intraperitoneal via) showed a similar anti-inflammatory effect to the reference drug, dexamethasone at 1.5 and 3.0 mg/kg, and the LuAc/ dexamethasone mixture (12.5/1.5 mg/kg) showed a better effect than dexamethasone alone, in this model. In addition, LuAc at 1.0, 10 and 20 mg/kg exhibited a better activity than pentoxifylline (1 and 25 mg/kg) on carrageenan-induced neutrophils migration; it also inhibited the myeloperoxidase activity at 0.1, 1, 10 ug/mL on ILhuman neutrophils respect to Ind (10 μg/mL); this inhibition was dose-dependent. In this assay, also described that LuAc (1, 10, 25 and 50 μg/mL) inhibited liberation of lactate dehydrogenase, and this effect was better than Titon X-100 (0.2%, a cytotoxic drug). The author suggests that the action mechanism of LuAc as antiinflammatory agent is due to decreased production of iNOS, and inhibited pro-inflammatory cytokine levels [29].
Anti-inflammatory activity in chronic paw edema model induced with CFA
In this assay, CFA was used to induce the chronic inflammatory process, and PBZ as reference drug. In Figure 5 are describes the results. The anti-inflammatory effect of LuAc administered for three weeks (from day 7 to day 28) was similar to the reference drug (PBZ, 100 mg/kg) during experimental period. At days 15, 21 and 28, the inhibitory effect was very similar; being these values 31.58, 40.15 and 42.94 % of inhibition by PBZ, respectively, and 33.39, 41.38 and 42.75% by LuAc (at 125 mg/kg). Through this experiment, it was shown that the anti-inflammatory activity of LuAc is very similar to the reference drug (PBZ). Previously, it has been described that LuAc at 50 mg/kg showed anti-arthritic effect on collagen-induced arthritis assay (DBA/1J mice). In this model, LuAc suppressed the expression of inflammatory cytokines in serum, such as TNF-alpha, IL-1β and 18F-fluorodeoxyglucose, and decreased the osteoclastogenesis in in vitro assay using bone marrow-derived macrophages stimulated with lipopolysaccharide [9]. Kweifio-Okai and Carroll [4] described the anti-arthritic effect of the LuAc (isolated from Alstonia booneri) at 66 mg/kg, assayed in CFA-induced arthritic test in male Wistar rats. In that case, LuAc (66 mg/kg) was administered orally every 48 h from day 32 at day 40 post-adjuvant administration. At day 60, the serum from the treated rats were extracted and then some biochemical parameters were determined. LuAc restored the alkaline phosphatase to normal levels, and also reduced creatinine (22%), as well as the fact that the spleen and liver weight did not increase with respect to arthritic rats.
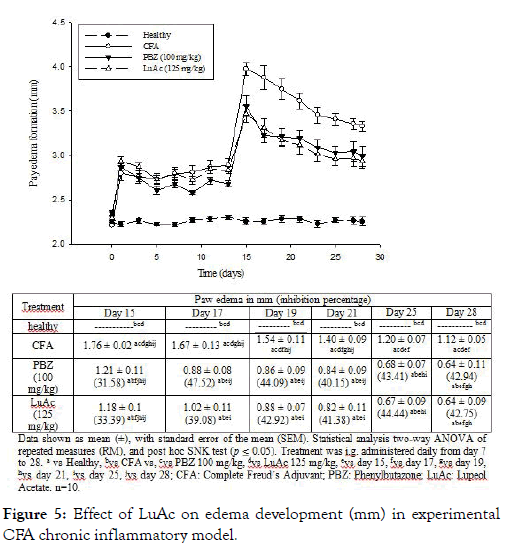
Figure 5: Effect of LuAc on edema development (mm) in experimental CFA chronic inflammatory model.
During this CFA assay, a BW gain was registered in all groups, the results are described in Figure 6. In this case, the BW gain was less in PBZ and LuAc groups with respect to healthy group. Even so, this BW gain was lower than the CFA group. At day 15, the BW gain was 1.8 g (healthy), 0.2 (CFA), -0.5 (PBZ) and -0.4 g (LuAc), and at day 28, these values were 2.4 (healthy), 1.1 (CFA), 0.6 (PBZ) and 0.5 g (LuAc). This poor BW gain in PBZ and LuAc groups was most evident from day 15 to day 28. At the end of experimental period, the BW gain between PBZ and LuAc groups was very similar.
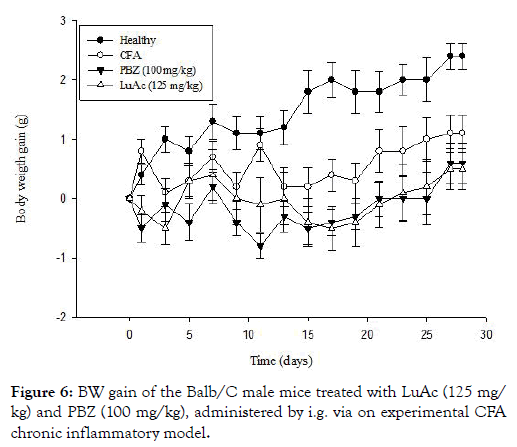
Figure 6: BW gain of the Balb/C male mice treated with LuAc (125 mg/kg) and PBZ (100 mg/kg), administered by i.g. via on experimental CFA chronic inflammatory model.
The BW gain has been used in recent works as an indirect parameter that helps to measure the establishment and development of chronic inflammatory process. In this model, the BW gain is poor and slow due to the mice losing weight due to anorexigenic effect (lack of food consumption) because they cannot move; in addition, the neuropeptide Y/leptin axis is inhibited, also showing hyperalgesia, allodynia and cachexia [24,30]. The results on the BW gain and the anti-inflammatory effect of LuAc group (at 125 mg/kg) were very similar to PBZ (100 mg/kg). In this case, the lost weight was very similar between PBZ and LuAc, but this BW gain was below that of CFA group and even lower than the control group. To date, the effect of LuAc on BW gain of mice with chronic CFA-induced inflammation has not yet been described.
In addition, in popliteal lymphoid node (taken from the right hind paw) of all groups, CD3+, CD4+ and CD8+ lymphocytes were quantified by staining and flow cytometry method. The results are described in Figure 7. CD3+ lymphocytes in CFA group were higher and in the PBZ and LuAc groups these values were low, this value being lowest in PBZ group. Similar behavior was observed between CD3+/CD4+ and CD3+/CD8+ relationship. The CD3+ and CD4+ levels were very similar to the reference drug (PBZ). Of the few works carried out in vivo model (such as collagen-induced arthritis in DBA 1J mice) with LuAc as substances with anti-arthritic effect, to date there is only one report that describes the effect of this compound on the levels of CD4+, CD25+, FOXP3+ cells, where cells were quantified in spleen and lymph node; LuAc also reduced the NF-kB, COX-2 levels. In this case, LuAc and liposomal of LuAc showed an anti-arthritic effect in in vitro and in vivo assays. In vitro assay (RAW 264.7 cell line) found that LuAc reduced osteoclastogenesis; it also inhibited the release of TNF-alpha, IL 1β, IL-17 and IL-6, while reducing the NF-kB activity, and showed a good anti-inflammatory response. This effect was better than curcumin, used as reference control [3].
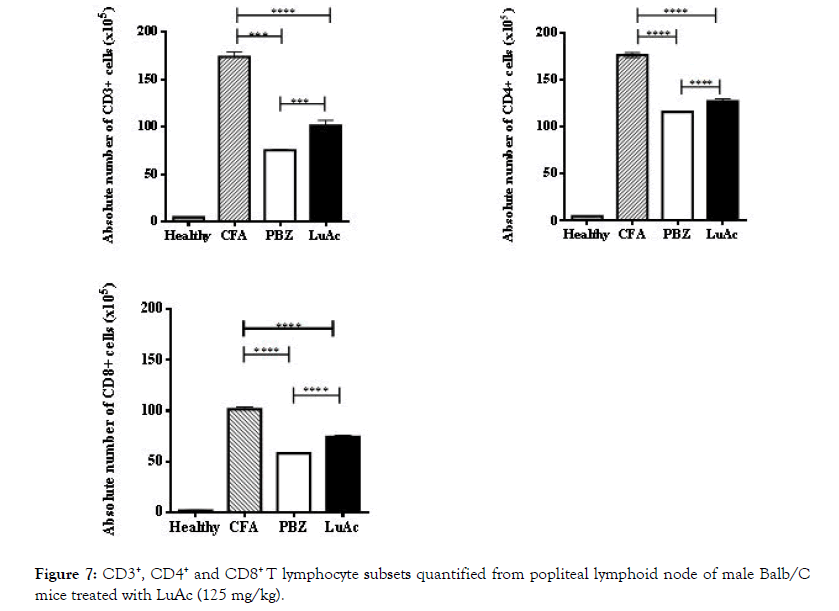
Figure 7: CD3+, CD4+ and CD8+ T lymphocyte subsets quantified from popliteal lymphoid node of male Balb/C mice treated with LuAc (125 mg/kg).
At the end of CFA experiment (day 28), COX-2 activity was also quantified. This enzyme was quantified in popliteal lymphoid node, and the results are described in Figure 8. In this case, LuAc (125 mg/kg) caused a significant decrease in the values with respect to CFA group (8.99 ± 0.019 vs 11.37 ± 0.012 IU/mg protein), this value was even lower than that shown by the reference drug, PZB (10.14 ± 0.030 IU/mg protein). The effect of the LuAc (125 mg/ kg) on COX-2 activity was slightly better than PBZ (100 mg/kg). It should be noted that PBZ is a non-steroidal anti-inflammatory drug, COX-2 inhibitor, and inhibited the biosynthesis of prostaglandins. In a previous paper, it was described that LuAc reduced COX-2 activity, employing the same model (experimental CFA chronic inflammation model) [3].
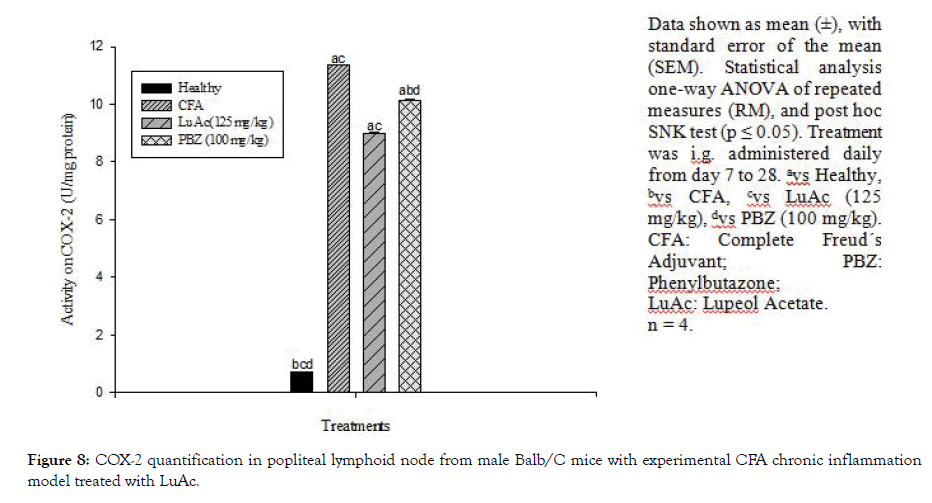
Figure 8: COX-2 quantification in popliteal lymphoid node from male Balb/C mice with experimental CFA chronic inflammation model treated with LuAc.
In addition, the histological analysis was made of the right rear leg joint, soft tissue, popliteal lymphoid node and liver of the male mice treated with LuAc and PBZ (Figures 9-12). Joints and soft tissue from healthy mice did not show any type of alteration; however, the CFA group showed sinusoidal hyperplasia in the joint as well as an increase in soft tissue size caused by the CFA-induced inflammatory process. LuAc and PBZ groups did not show any alteration on the paw joint and on the synovial membrane, but a significant increase in soft tissue size was observed with respect to healthy group (Figures 9 and 10). In soft tissue, a significant increase in leukocyte infiltrate was observed as a result of the chronic inflammatory process induced with CFA; in LuAc and PBZ groups the leukocyte infiltrate was lower. It is important to describe that the Rx analysis of the joints showed that at the microscopic level there was no significant alteration in the joint or in the synovial membrane; so, this biological test (CFA chronic inflammation model) allows us to evaluate mainly the anti-inflammatory effect of natural or synthetic substances.
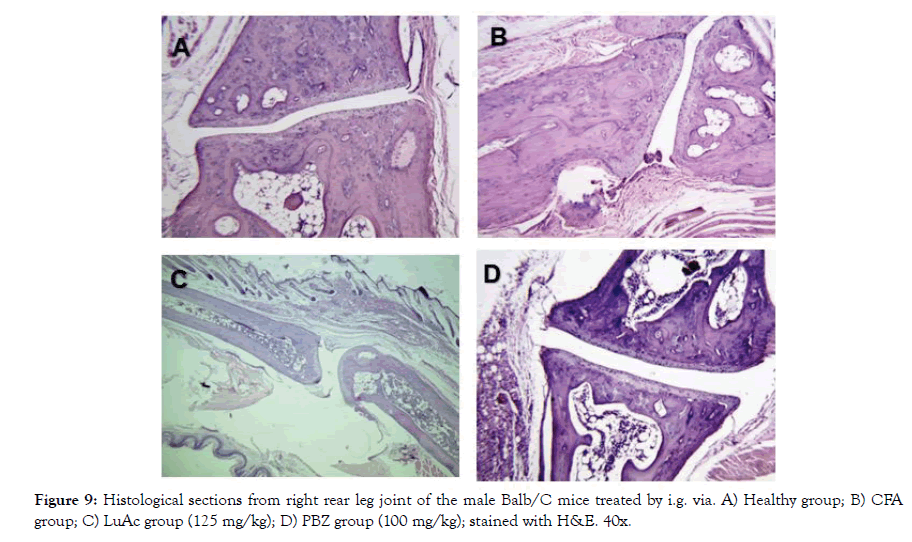
Figure 9: Histological sections from right rear leg joint of the male Balb/C mice treated by i.g. via. A) Healthy group; B) CFA group; C) LuAc group (125 mg/kg); D) PBZ group (100 mg/kg); stained with H&E. 40x.
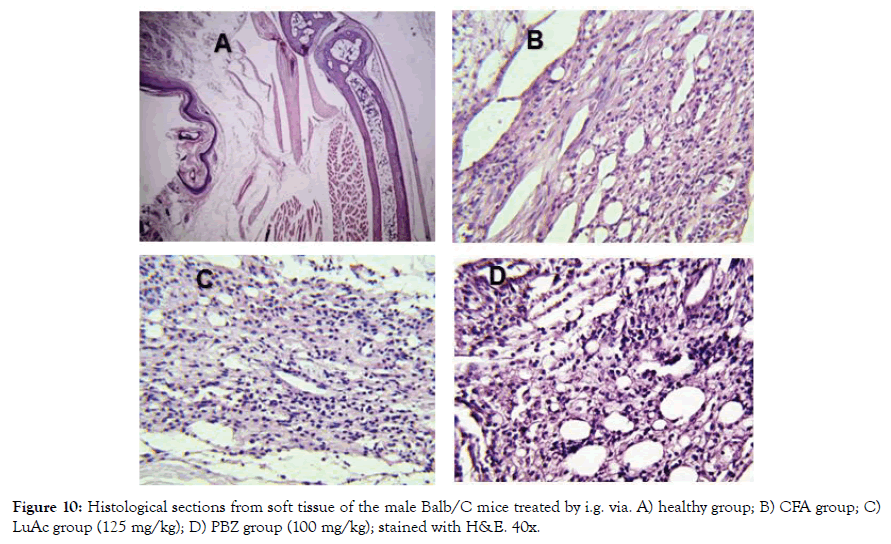
Figure 10: Histological sections from soft tissue of the male Balb/C mice treated by i.g. via. A) healthy group; B) CFA group; C) LuAc group (125 mg/kg); D) PBZ group (100 mg/kg); stained with H&E. 40x.
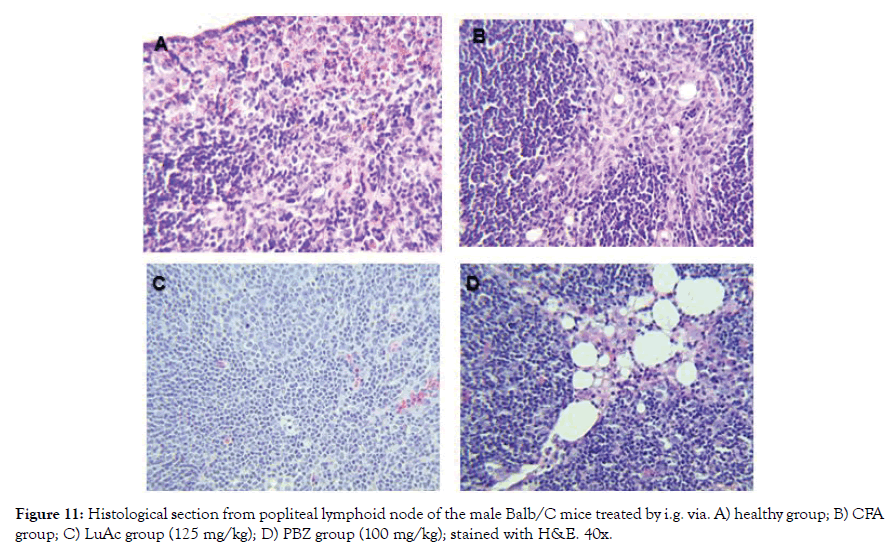
Figure 11: Histological section from popliteal lymphoid node of the male Balb/C mice treated by i.g. via. A) healthy group; B) CFA group; C) LuAc group (125 mg/kg); D) PBZ group (100 mg/kg); stained with H&E. 40x.
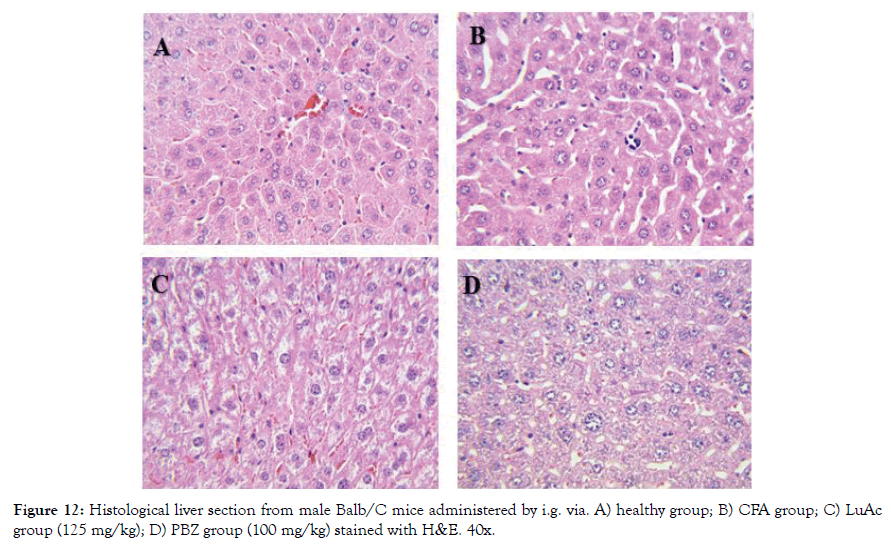
Figure 12: Histological liver section from male Balb/C mice administered by i.g. via. A) healthy group; B) CFA group; C) LuAc group (125 mg/kg); D) PBZ group (100 mg/kg) stained with H&E. 40x.
In Figure 11 (A-D) a histological section of popliteal lymphoid node of mice (from right leg) is shown; healthy group showed a normal architecture without change; the AFC group presented sinusoidal hyperplasia (infiltration of cells in sinusoids space), the same effect was presented by the PBZ group (reference drug) and the LuAc group presented a slight leukocyte infiltration. In CFA model, LuAc and PBZ groups presented many vacuoles due to CFA vehicle, these were more evident in PBZ group. In LuAc group, a decrease in leukocyte was observed, this decrease was better than PBZ group.
Finally, in Figure 12 (A-D) is showed the histological section from liver. The healthy and LuAc groups showed no alteration in hepatocytes; on the other hand, the CFA group showed a slight microabscess and scarce hematopoiesis and in the PBZ group only scarce steatosis was observed. It is well known that more 1100 drug cause liver damage, such as steatosis, fibrosis, hepatocarcinoma [31]. In this study, we found that PBZ causes a mild steatosis when administered by oral via during two weeks, damage that does not cause LuAc at 125 mg/kg when it was administered for the same time and via.
Through this work, we reaffirm the fact that LuAc is a promising molecule for developing a useful medication in chronic inflammatory processes such as RA and OA. It should be noted that the majority of anti-inflammatory agents provoke various adverse effects, such as immunosuppression, cardiovascular and hepatic alterations. Given the potential of LuAc, a biotechnical process was recently published to obtain LuAc form the leaves of C. chayamansa [21]. Currently, additional studies are underway to determine the acute and subacute toxicity in vivo of LuAc, as well as its anti-inflammatory effect when using a mixture of LuAc with Ind, PBZ or naproxen in CFA model.
Cnidoscolus tehuacanensis is an endemic medicinal plant and is potential source to obtain LuAc, it also contains α- and β-amyrin acetate in good quantities. In the model of CFA-induced chronic inflammation, LuAc (at 125 mg/kg) showed a good antiinflammatory activity, similar effect to PBZ. In addition, this compound as well as PBZ favored BW gain.
On the other hand, the LuAc inhibited of COX-2 activity and showed a slight leukocyte infiltration in liver tissue, these effects were better than those of PBZ. LuAc did not cause steatosis in the liver of animals treated for two weeks in CFA-induced chronic inflammation model. The Cteh extract showed a good leishmanicidal activity, but the LuAc showed a poor activity.
All authors have read and approved the final version of the manuscript and declare that they have no conflicts of interest.
This study was supported by a grant from IMSS Project CNIC R-2017-785-059. The authors thank Susan Drier-Jonas for English assistance with the manuscript
Citation: Juárez-Vázquez MDC, Siordia-Reyes GA, Pérez-González MZ, Chávez-Rueda KA, Legorreta-Haquet MV, Nieto-Meneses R, et al. (2020) Leishmanicidal and anti-inflammatory activities of Lupeol Acetate isolated from Cnidoscolus tehuacanensis Breckon. Med Aromat Plants (Los Angeles) 9: 347. doi: 10.35248/2167-0412.20.9.347
Received: 14-Jan-2020 Accepted: 25-Apr-2020 Published: 02-May-2020 , DOI: 10.35248/2167-0412.20.9.347
Copyright: © 2020 Juárez-Vázquez MDC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.