
Journal of Clinical Toxicology
Open Access
ISSN: 2161-0495

ISSN: 2161-0495
Case Report - (2021)Volume 11, Issue 3
Calcium Channel Blockers (CCBs) may cause profound vasopressor resistant hypotension in case of intoxication. Recently, the EXTRIP workgroup recommended against the use of Extra Corporeal Treatment Modalities (ECTM) for the elimination of amlodipine. Anecdotal evidence suggests that Veno-Arterial Extra Corporeal Membrane Oxygenation (VA-ECMO) may be beneficial in patients with severe shock due to amlodipine intoxication.
We report a case of severe amlodipine poisoning (total amlodipine intake 625 mg), in which we used awake-VA- ECMO support for hemodynamic stabilization. A combination of a HCO dialyzer and the Cytosorb® adsorber was used to eliminate amlodipine in two consecutive treatment sessions. Amlodipine dialyzer clearance and Cytosorb® adsorber clearance were 13.8 (-2,3–20.4) mL/min and 17.5 (9.6–25.6) mL/min, respectively. Clearance levels varied depending on drug plasma levels. During this treatment, initial amlodipine levels fell by 48% and 15% with a total eliminated amount of 242 and 352 μg of amlodipine, respectively, in the dialysate. EXTRIP criteria for dialyzability could not be met by the investigated modalities. ECMO therapy was discontinued after three days and the patient fully recovered.
In conclusion, VA-ECMO support can be used for hemodynamic stabilization of patients with severe amlodipine poisoning, while the extracorporeal elimination techniques used in this setting were unable to remove a meaningful amount of drug.
Amlodipine intoxication; VA ECMO therapy; HCO dialysis; Cytosorb treatment
Calcium Channel Blockers (CCBs) are the leading cardiovascular drug class involved in intoxications [1]. In case of overdose, CCBs cause profound hypotension that is frequently resistant to vasopressor treatment. First line treatment focuses on general measures such as activated charcoal treatment. Calcium as well as dopamine and norepinephrine may improve hemodynamic parameters [2]. Anecdotal evidence suggests that Veno-Arterial Extra Corporeal Membrane Oxygenation (VA-ECMO) may be beneficial in patients with severe shock or cardiac arrest due to amlodipine intoxication [3]. Despite its low molecular size (408.9 D), amlodipine is considered non-dialyzable due to its high plasma protein binding (97%) and large volume of distribution (20 L/kg). Treatment options like therapeutic plasma exchange have been used anecdotally [4]. Recently, the EXTRIP workgroup recommended against the use of Extra Corporeal Treatment Modalities (ECTR) for the elimination of amlodipine [5]. However, data regarding the use of modern High-Cut-oOff (HCO) dialyzers, which may eliminate highly protein bound substances [6], in combination with a Cytosorb® (CytoSorbents, Corporation, New Jersey, USA) adsorber, which is known to bind middle-sized molecules by nonspecific hydrophobic interactions, is limited.
We here report a case of life-threatening amlodipine intoxication, in which we used VA-ECMO for hemodynamic stabilization and drug elimination using a HCO dialyzer and Cytosorb® adsorber.
A 27-year-old woman was transferred to our tertiary care hospital 18 hrs after ingestion of 625 mg of amlodipine, 700 mg of losartan and 175 mg of hydrochlorothiazide in a suicidal attempt. The patient had no known prior somatic illnesses but had already attempted suicide eleven years earlier. Despite administration of fluids, high-dose vasopressors and hyperinsulinemic euglycemia therapy in the referring hospital the patient further deteriorated. On admission to our intensive care unit, she displayed the following vital signs: mean arterial blood pressure 57 mmHg whilst on high- dose norepinephrine therapy (0.41 μg/kg/min), heart rate 112 bpm, respiratory rate 20/min and a temperature of 36.4°C, pulse oximetry displayed 98% oxygen saturation with 6 L/min oxygen supplement. The patient was slightly lethargic and had facial swelling most probably due to the side effects of the amlodipine overdose. She had developed Acute Kidney Injury (AKIN 3); her serum creatinine was 239 μmol/L.
Despite an increase of the norepinephrine dose of up to 1.06 μg/kg/min, blood pressure further deteriorated. VA-ECMO was initiated to support circulation and to prevent further organ failure. The blood pressure stabilised immediately after initiation of the ECMO support.
We initiated dialysis therapy with a HCO filter (APS21 EH, Asahi, polysulphone membrane, 1.8 m²) in combination with a Cytosorb® adsorber soon after. Both columns were connected in series. Amlodipine plasma levels were measured by LC-MS/MS (gradient reverse-phase HPLC followed by ion detection in a API4000 triple- quadrupole mass spectrometer using deuterated amlodipine as the internal standard) during two consecutive dialysis sessions (Figure 1) with a treatment time of 1405 and 1235 min (blood flow 150 mL/min, dialysate flow 75 mL/min). Median amlodipine dialyzer clearance was 13.8 (-2,3–20.4) mL/min. Cytosorb® plasma clearance was 17.5 (9.6–25.6) mL/min with a combined plasma clearance of 34.9 (26.1–44.2) mL/min. Extra Corporeal Treatment (ECTR) clearance rates differed over time according to amlodipine plasma levels without any measurable clearance below 150 μg/L (Figure 2). A total amount of 242 and 352 μg amlodipine was found in the total spent dialysate. During the treatments we measured a relative reduction in the amlodipine plasma levels of 48% and 15%, respectively (Figure 3).
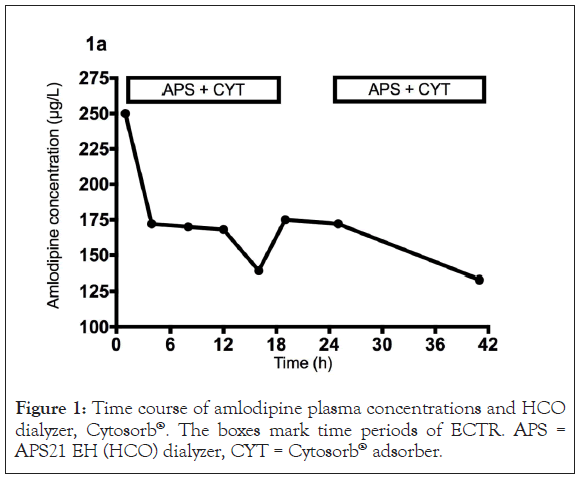
Figure 1: Time course of amlodipine plasma concentrations and HCO dialyzer, Cytosorb®. The boxes mark time periods of ECTR. APS = APS21 EH (HCO) dialyzer, CYT = Cytosorb® adsorber.
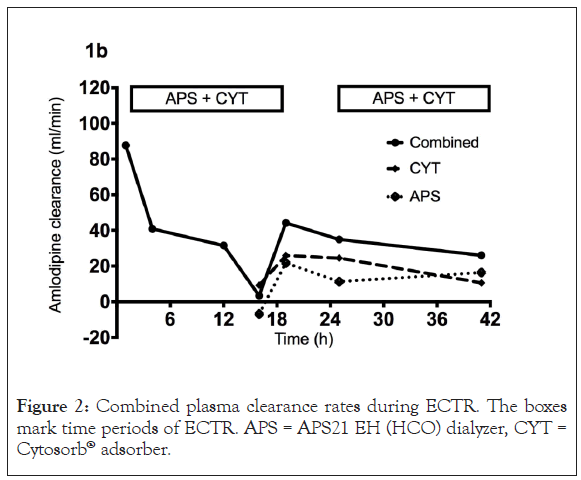
Figure 2: Combined plasma clearance rates during ECTR. The boxes mark time periods of ECTR. APS = APS21 EH (HCO) dialyzer, CYT = Cytosorb® adsorber.
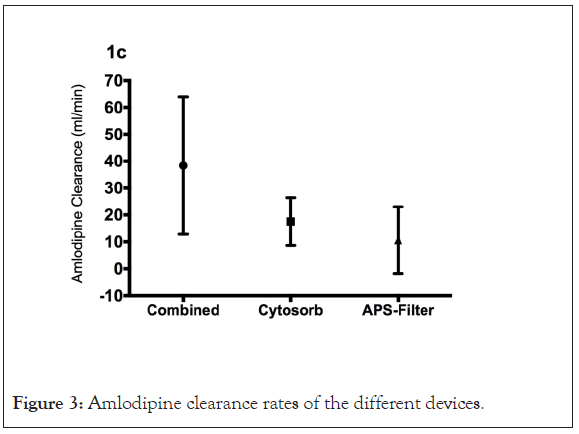
Figure 3: Amlodipine clearance rates of the different devices.
Dialysis and Cytosorb® therapy continued for 48 hrs and during this time the need for vasopressor therapy resolved and We were able to achieve a negative fluid balance by ultrafiltration. The patient was weaned from ECMO after three days and had an uneventful recovery. Relevant case report characteristics recommended by the EXTRIP (Extra Corporeal Treatments In Poisoning) workgroup [7] are summarized in Table 1, results are given as median (IQR).
| General information | Case |
|---|---|
| Age (y) | 27 |
| Weight (kg) | 82 |
| Height (cm) | 164 |
| Gender | ♀ |
| Concurrent diseases | none |
| Source providing the history of the poisoning | Patient |
| Time from ingestion to hospital admission (h) | 18 |
| Known comedication | none |
| Other toxins | Losartan, HCT |
| Activated charcoal given | no |
| ICU stay (d) | 6 |
| Discharge from hospital (d) | 10 |
| Laboratory values | |
| Albumin (g/L) | 34 |
| Creatinine at baseline (µmol/L) / eGFR acc. CKD EPI (mL/min/1.73m2) | 239/23 |
| Plasma amlodipine peak level (µg/L) | 250 |
| Urine excretion (ml/d) | 6000 |
| Urine amlodipine concentration (µg/L) | NA |
| Hematocrit (%) | 27.8 |
| ECTR characteristics | |
| Modality of ECTR | CVVHD |
| Indication for ECTR | Detoxification, AKI |
| ECTR start after admission (h) | 1 |
| ECTR device | HCO Dialyzer (APS21 EH) Asahi (polysulphone, 1.8m²)+Cytokine adsorber (Cytosorb® ) |
| ECTR time (min) | 1105/1235 |
| Blood flow (mL/min) | 150/150 |
| Dialysate flow (mL/min) | 75/75 |
| Ultrafiltration rate (mL/h) | 50/114 |
| Anticoagulation | Heparin |
| Full body amlodipine reduction ratio during ECTR (%)* | 48/15 |
| ECTR amlodipine plasma clearance (mL/min)** | 34.9 (26.1–44.2) |
| Total amount of amlodipine in the collected dialysate (µg) | 342/252 |
| Note: *Calculations of reduction ratios were executed according to equation 1. | Negative |
Note: *Calculations of reduction ratios were executed according to
equation 1.
Eq. 1: RR = (Cpost – Cpre)/Cpre × 100
**Dialyzer clearance was measured according to equation 2, using
the patients’ hematocrit concentration (Hct) at the time of clearance
sampling:
Eq. 2:Kplasma = QB x (1 – Hct/100) x ((Cart – Cven)/Cart)
Table 1: Relevant case report characteristics recommended by the EXTRIP workgroup.
During standard dosing, the therapeutic range of amlodipine is 6-24 μg/L [8]. A fatal intoxication was reported after ingestion of 70 mg amlodipine with peak serum levels as low as 185 μg/L [9]. The highest reported amlodipine plasma level measured at autopsy was 2700 μg/L after the ingestion of 140 mg [10]. In our patient the ingested amount of amlodipine (625 mg) as well the peak drug level (250 μg/L) were in the range for which the mortality risk is very high. After the implementation of all recommended first line measures for the management of CCB poisoning, the patient continued to deteriorate, so we initiated VA-ECMO support as rescue measure to maintain tissue and organ perfusion by improved hemodynamics.
Amlodipine is generally considered not dialyzable [5]. Although other extracorporeal methods like therapeutic plasma exchange [11,12] and the Molecular Adsorbent Recirculating System (MARS) [13] have been employed in case reports, substantial removal of the substance could never been shown. As our patient suffered from acute kidney injury requiring renal replacement therapy, we used a HCO dialysis membrane in combination with a Cytosorb® filter to achieve a possible amlodipine elimination. Although the drop in amlodipine plasma levels and the extracorporeal clearance of the HCO-membrane and the Cytosorb® cartridge suggests that a removal of amlodipine takes place, one must be cautious in interpreting these results. Interestingly, the amlodipine clearance, achieved during Cytosorb® and HCO therapy, showed a close correlation with the absolute amlodipine plasma levels. This may reflect that higher concentrations of unbound substance molecules lead to a better elimination by ECTR. However, according to the EXTRIP workgroup guideline methodology a compound is considered to be dialyzable if >30% of the substance can be removed by a six hour treatment. If this primary criterion cannot be determined, a dialysis clearance per total clearance of >75% or a half-life during dialysis per half-life without dialysis of <25% must be achieved [14].
Even with initial high amlodipine plasma concentrations amlodipine half-life in this case (46 hrs) was not significantly reduced compared to the standard elimination half-life (40 hrs) reported in the literature. With a total amount of 242 and 352 μg in the spent dialysate, the total eliminated amount of amlodipine will most likely not exceed 3% of the ingested 625 mg, which is considered to be the lower threshold for slight dialyzability according to EXTRIP criteria. Therefore, in accordance to recent EXTRIP recommendations, we cannot recommend routine HCO- dialysis membranes in combination with Cytosorb® for the purpose of amlodipine elimination.
This work was supported by the “HILF” program of the Hannover Medical School.
Citation: Schmidt JJ, Busch M, David S, Kühn-Velten WN, Hoeper MM, Kielstein JT (2021) Life-Threatening Amlodipine Overdose Requiring ECMO Support Treated by High-Cut-Off Dialysis and Cytosorb® . J Clin Toxicol. 11:479.
Received: 25-Mar-2021 Accepted: 08-Apr-2021 Published: 15-Apr-2021 , DOI: 10.35248/2161-0495.21.11.479
Copyright: © 2021 Schmidt JJ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.