Medical & Surgical Urology
Open Access
ISSN: 2168-9857
+44-77-2385-9429
ISSN: 2168-9857
+44-77-2385-9429
Case Report - (2019)Volume 8, Issue 4
Introduction: Nephroblastoma or Wilms’ Tumor (WT) is the most common kidney tumor in children. While its incidence is close to 8 cases per million children, it is only 0.2 cases per million adults. In fact, no more than 300 cases have been described in the English or French literature. And while the treatment of nephroblastoma is very well codified in children, its rare incidence in adults leads to a lack of specific treatment regimen.
Observation: A 28-year-old female, with no medical history, presented with right flank pain for 4 months. Clinical examination revealed a palpable kidney. CT scan found a large heterogeneous renal mass measuring 15 × 14 × 11 cm without evidence of adjacent structure ’ s infiltration. The patient underwent an open transperitoneal radical nephrectomy. Pathologic examination revealed a nephroblastoma. Due to lack of financial means, she was unable to have adjuvant chemotherapy. A control CT scan performed at 6 months revealed multiples recurrences. The patient died before being able to benefit from chemotherapy.
Conclusion: This case illustrates the importance of adjuvant chemotherapy, despite R0 surgery in a localized nephroblastoma. The key to treat nephroblastoma is, first, nephrectomy, then chemotherapy, whatever the stage. Staging only plays a role in the chemotherapy protocol.
Wilms’ tumor; Nephroblastoma; Nephrectomy; Chemotherapy
Nephroblastoma or Wilms’ Tumor (WT) is the most common kidney tumor in children. In 90% of cases, patients are under 7 years of age at diagnosis [1]. While its incidence is close to 8 cases per million children, it is only 0.2 cases per million adults. In fact, no more than 300 cases have been described in the English or French literature [2]. And while the treatment of nephroblastoma is very well codified in children, its rare incidence in adults leads to a lack of specific treatment regimen.
A 28-year-old female, with no medical history, presented with right flank pain for 4 months, without haematuria or other associated symptoms. Clinical examination revealed a palpable kidney. Laboratory investigations didn’t show nor anemia, nor acute kidney failure, but a microscopic haematuria. CT scan found a large heterogeneous renal mass measuring 15 × 14 × 11 cm, containing necrosis areas, pushing forward the adjacent structures, without evidence of infiltration, nor inferior veina caval thrombosis, with latero-aortic adenomegalies (Figure 1). No distant metastasis was found.
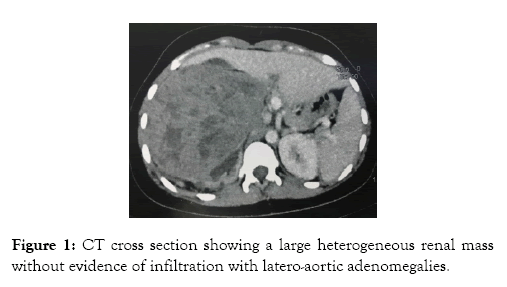
Figure 1: CT cross section showing a large heterogeneous renal mass without evidence of infiltration with latero-aortic adenomegalies.
Pathologic examination revealed a nephroblastoma, with triphasic cellular pattern (blastemal, epithelial and stromal with rhabdomyoblastic differentiation cells), and presence of lymphvascular invasion. The surgical margins were clear. The ganglia (four) and adrenal gland were free from tumoral invasion. Given the NWTSG classification, it was a Stage I.
The patient underwent an open transperitoneal radical nephrectomy, with hilar and latero-aortic lymphadenectomy. The tumor capsule was intact. There was no infiltration of the renal vein’s wall (Figure 2). The postoperative course was simple.
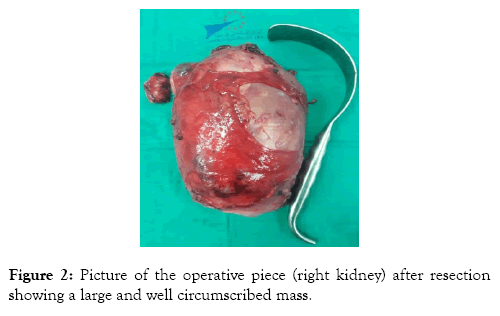
Figure 2: Picture of the operative piece (right kidney) after resection showing a large and well circumscribed mass.
We referred the patient to oncology department, but due to lack of financial means, she was unable to have adjuvant chemotherapy. A control CT scan performed at 6 months revealed the presence of cannonball lung metastases, contralateral renal and multiple hepatic localizations, tumor invasion of the inferior veina cava wall and multiple retroperitoneal lymphadenopathies (Figure 3). Once again referred to oncology, the patient died before being able to benefit from chemotherapy.
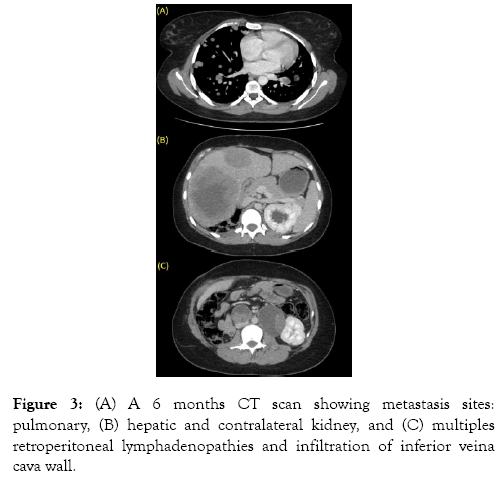
Figure 3: (A) A 6 months CT scan showing metastasis sites: pulmonary, (B) hepatic and contralateral kidney, and (C) multiples retroperitoneal lymphadenopathies and infiltration of inferior veina cava wall.
Nephroblastoma or Wilms’ Tumor (WT) is the most common kidney tumor in children. In 90% of cases, patients are under 7 years of age at diagnosis [1]. It is rare in adults (0.5% of all renal tumors, 0.2 cases per million adults per year in USA and Europe), and often diagnosed in advanced or metastatic stages [2]. It is derived from totipotent metanephric blastema cells that don ’ t differentiate into glomeruli and tubules [3]. Several chromosomal alterations (WT1 gene or 11p13, 11p15, 11q, 16q, 7p) have been found in patients with Wilm's tumors, suggesting that this is a multigenic disease [4,5].
Unlike in children, where the main reason for consultation is the discovery of a painless palpable mass, the adult consults most often for low back pain and/or hematuria, which is a symptomatology common to all renal masses [3,6]. The association with general signs such as asthenia or weight loss leads to fear an advanced disease [2]. It is thus difficult to clinically suspect a nephroblastoma in an adult. However, some authors recommend suspecting Wilms’ tumor in young adults who consults for low back pain with a clinically palpable and rapidly growing mass [1]. Three types of cell are found in Wilms' tumor, in varying proportions: blastemal, stromal, and epithelial. The proportion and the degree of maturation of these components vary significantly, making the histological appearance of each tumor unique [7]. However, the presence of all three types in a same tumor is rare [3]. Thus, it is not necessary to have the 3 types to make the diagnosis [2]. Unlike epithelial- and stromal-predominant tumors, blastemal – predominant Wilms ’ tumors have a poorer prognosis [8]. Another element to look for is an anaplasia: more spread is the anaplasia, higher-risk is the tumor, and poorer is the prognosis [9]. Classical triphasic WT is easily diagnosed by pathologists, but when only one component is present or massively predominant, the differential diagnosis may be more difficult (Table 1) [1,7]. In case of doubt or difficult diagnosis, immunohistochemical staining can help by searching presence of cytokeratin, vimentin, desmin, actin, and WT1 [8]. Kilton, Mathews, and Cohen published in 1980 six criteria to help diagnosis of Wilms’ tumor in adult (Table 2) [10].
| Mainly epithelial differentiation | Mainly stromal differentiation | Mainly blastemic differentiation |
|---|---|---|
| - Metanephric adenoma - Renal cell carcinoma |
- Clear cell sarcoma of the kidney - Mesoblastic nephroma - Synovial sarcoma |
- Lymphoma - PNET - Ewing Sarcoma) - Rhabdomyosarcoma - Metastatic small cell tumors |
Table 1: Histology differential diagnosis.
| 1) The tumor under consideration should be a primary renal neoplasm |
| 2) Presence of primitive blastemic spindle or round cell component |
| 3) Formation of abortive or embryonal tubules or glomerular structures |
| 4) No area of tumor diagnostic of renal cell carcinoma |
| 5) Pictorial confirmation of histology |
| 6) Patient's age >15 years. |
Table 2: Criteria of Kilton, Mathews and Cohen.
Radiographically, in adults, there are no specific and characteristic signs of nephroblastoma. There is also no evidence of radio-histological correlation, making differential diagnosis difficult with renal cell carcinomas [1]. At CT scan, it is most often a large illdefined heterogeneous mass, with hypodense areas, slightly enhanced after contrast injection compared to the rest of the renal parenchyma [3,6,9].
The most common sites of metastasis are lung, liver, bones, skin, bladder, colon, brain and contralateral kidney [11,12]. A metastasis rate for adults is 29% at the time of diagnosis [11].
There are 2 groups that made guidelines on the management of Wilms Tumors in children: Children’s Oncology Group (COG, formerly National Wilms ’ Tumor Study Group) and Société Internationale d ’ Oncologie Pédiatrique (SIOP). Their classifications are based on the tumor extension, which is evaluated during the surgical procedure, on the resection and the imaging (Tables 3 and 4). However, the major difference lies in the therapeutic strategy. While SIOP recommends to realize chemotherapy 4 weeks (to 6 weeks if metastatic) before surgery, before having any histological confirmation, to reduce the tumor size and minimize the risk of intraoperative tumour rupture during resection [12], COG recommends to realize nephrectomy straight away after diagnosis [13]. However, this exposes to more complications, especially vascular and digestive wounds [14].
| Stage I | Tumor limited to kidney and completely resected |
| Stage II | Tumor extends beyond kidney (penetration beyond pseudo-capsule, peri-aortic node involvement, renal vessel involvement beyond kidney) but completely resected. |
| Stage III | Residual non-haematogenous tumor confined to abdomen, e.g. tumor rupture before or during surgery, tumor not completely resectable, and nodes beyond peri-aortic chain involved. |
| Stage IV | Haematogenous metastasis (Lungs, liver) |
| Stage V | Bilateral renal involvement. |
Table 3: SIOP Staging [12].
| Stage I | Tumor limited to kidneys, completely resected. - Renal capsule is intact - Tumor not ruptured or biopsied prior to removal - Vessels of the renal sinus are not involved - No evidence of tumor present at or beyond the margins of resection |
| Stage II | Tumor completely resected. - No evidence of tumor at or beyond the margins of resection - Tumor extends beyond the kidneys a residual (penetration of renal capsule, involvement of renal sinus) |
| Stage III | A residual, non-haematogenous tumor is present following surgery and confined to the abdomen. - Positive lymph nodes in the abdomen or pelvis noted - Penetration through the peritoneal surface is observed - Peritoneal implants noted - Gross or microscopic tumor remains postoperatively, including positive margins of resection - Tumor spillage is noted including biopsy - Tumor is treated with pre-operative chemotherapy - Tumor removed in more than one piece. |
| Stage IV | Haematogenous metastasis (lung, bone, liver, brain) or lymph nodes beyond the abdomen or pelvis noted. |
| Stage V | Bilateral renal involvement by the tumor at diagnosis. |
Table 4: COG/NWTSG Staging [13].
Given nephroblastoma represents more than 90% of the child's kidney masses, the strategy of the SIOP is legitimate in children. However, in the adult, because of the rarity of this tumor, there is no guideline. Thus, the strategy of the COG, more adapted, is the most adopted by the clinicians [1,3,8]. The principle of treatment is multimodality: surgery, chemotherapy and, eventually, radiotherapy. The choice of molecules, the duration, and the addition of radiotherapy depends on the NWTSG / COG stage (Table 5). The most effective molecules are: actinomycin D, vincristine, doxorubicin, cyclophosphamide, ifosfamide, etoposide and carboplatin. It should be emphasized that chemotherapy tolerance is lower in adults compared to children.
| COG/NWSTG Stage | Chemotherapy | Radiotherapy |
|---|---|---|
| I | Vincristine + Actinomycin 18 weeks |
- |
| II | Vincristine + Actinomycin 18 weeks |
- |
| III | Vincristine + Actinomycin + Doxorubicin 24 weeks |
10,8 Gy (Tumoral bed) |
| IV | Vincristine + Actinomycin + Doxorubicin 24 weeks |
+/- 12 Gy (if Lung) +/- 10,8 Gy (if residual tumor as Stage III) |
| V | Initial biopsy, chemotherapy and surgery at week 5 (Kidney preserving resection) |
|
Table 5: Treatment protocol given COG/NWTSG Staging [8].
The prognosis of the disease depends on tumor stage, pathological findings, and recurrence. The results for adults with Stage I or II disease are similar to those of children, with an overall survival of 82% [15]. However, since adults often have more advanced disease at the time of diagnosis, the prognosis is generally worse [3,8]. Histological elements of poor prognosis are blastema predominant type and diffuse anaplasia [12].
This case illustrates the importance of adjuvant chemotherapy, despite R0 surgery even in localized nephroblastoma (NWTG Stage I). Thus, the key to treat nephroblastoma in adults is, first, nephrectomy, then chemotherapy, whatever the stage. Staging only plays a role in the chemotherapy protocol, whether or not to use radiotherapy, and evaluate outcome.
Citation: Touzani MA, Othmane Y, Regragui S, Slaoui A, Zouaidia F, Karmouni T, et al. (2019) Localized Nephroblastoma in Adults: Is Surgery Enough? Case Report and Literature Review. Med Sur Urol 8:226. doi: 10.24105/2168-9857.8.226
Received: 12-Oct-2019 Accepted: 05-Dec-2019 Published: 13-Dec-2019 , DOI: 10.35248/2168-9857.19.8.226
Copyright: 2019 Touzani MA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.