Journal of Clinical and Experimental Ophthalmology
Open Access
ISSN: 2155-9570
ISSN: 2155-9570
Review Article - (2024)Volume 15, Issue 5
Topical ocular medications are traditionally considered to be first-line therapies for glaucoma and ocular hypertension. However, long-term therapy with topical medications can be difficult for the ocular surface to tolerate, has significant cost, results in frequent suboptimal patient compliance, and can adversely affect patient quality of life. Intracameral implants have been developed to overcome the physical barrier of the ocular surface by bypassing the cornea and conjunctival tissues for intraocular delivery. These drug delivery systems are designed to deliver therapeutic concentrations of medication in a continuous fashion to the target tissues. The implants have demonstrated durable intraocular pressure-lowering effects with favorable safety and varying key distinguishing characteristics. They have the potential to occupy an important position in the ophthalmologists’ armamentarium for treating glaucoma and ocular hypertension, including at earlier stages in the treatment journey.
Implant; Medication; Sustained-release; Pharmaceutical; Intervention; Glaucoma
Topical medical therapy has held the position of first-line therapy for more than 150 years, in part due to the fact that alternative treatments were less efficacious or carried more risk [1,2]. The most recent 2020 guidelines from the American Academy of Ophthalmology (AAO) still recommend topical medications for newly diagnosed Primary Open-Angle Glaucoma (POAG) or Ocular Hypertension (OHT) for most patients [3]. However, Selective Laser Trabeculoplasty (SLT) is now mentioned as an alternative first-line option for select patients. The evolution of laser technology from Argon Laser Trabeculoplasty (ALT) to SLT, the advent of Micro-Invasive Glaucoma Surgery (MIGS), and procedural pharmaceutical platforms have provided an opportunity to evolve beyond a reactive to an interventional approach to treating glaucoma. All three of these interventional categories can provide Intraocular Pressure (IOP) lowering while reducing or eliminating the need for chronic topical medications. Evidence to support the efficacy and safety of these interventions, coupled with their widespread availability, have prompted a reevaluation of the traditional topical-medication-first approach to favor a more proactive, targeted interventional glaucoma management pattern.
Traditionally, topical medical therapy has been the most common first-line treatment for lowering IOP. Prostaglandins, in particular, are efficacious, generally well-tolerated systematically, and only need to be administered once daily. However, long-term use of these agents is associated with adverse effects to the ocular surface such as dry eye, and to the periocular tissues such as periorbital orbitopathy. Adherence to therapeutic recommendations can be poor, leading to suboptimal IOP control and increased risk for progression and vision loss. In contrast, intracameral implants can administer medication directly to the target tissues, bypassing and therefore sparing the ocular surface. These drug delivery systems provide steady 24-hour control of IOP and eliminate the risk of under-treatment due to patient noncompliance. The implants can be placed in minimally invasive procedures and their usage can reduce or eliminate the need for topical drops.
The present article reviews the pros and cons of topical medications versus intracameral procedural pharmaceuticals for the treatment of glaucoma and ocular hypertension. It draws upon manuscripts published in the past 10 years, using the PubMed search terms adherence, glaucoma, side effects, Durysta, and iDose.
Topical medications
Despite recent advancements in surgical interventions, topical medications are the most commonly prescribed first-line treatment for most forms of glaucoma. A wide array of topical medications are available with different mechanisms of action for lowering IOP such as increasing uveoscleral and/or trabecular outflow or decreasing aqueous humor production. Formulations are available that contain two different classes of medication with complementary mechanisms of action. In addition, topical medications are associated with relatively few systemic side effects. Prostaglandin Analogs (PGAs) are the most frequently prescribed topical drops due to their being some of the most efficacious and systemically well tolerated glaucoma medications. PGAs also provide the convenience of once-daily dosing.
Despite these attributes, a number of limitations make topical medications a suboptimal solution for a chronic disease such as glaucoma. These limitations can be broadly categorized as cellular changes, patient side effects, and adherence issues with associated risk of progression. On a cellular level, the ocular surface is a barrier to the molecules in medications [4]. As a result, drug concentrations in topical medications tend to be high, which can lead to unwanted systemic, ocular, and periocular side effects [5,6]. Chronic exposure to topical medications, along with their associated preservatives, cause changes in goblet cell populations of the conjunctiva [7]. Fixed combination medications can lower this preservative load on the ocular surface, and certain preservative-free formulations are now available; however, these are often costlier, and long-term, randomized controlled trials comparing them to preserved medications are lacking [8].
Benzalkonium chloride, the most common preservative, is effective at maintaining aseptic conditions in the medication bottle, but the compound has numerous deleterious effects on ocular cells and tissues such as tear film instability, loss of goblet cells, conjunctival squamous metaplasia and apoptosis, disruption of corneal epithelial tight junctions, and damage to deeper ocular tissues. Mechanisms for these effects include mitochondrial dysfunction, release of proinflammatory cytokines, apoptosis, oxidative stress, as well as direct interactions with lipids in the tear film and cell membranes [9].
These pathologic cellular changes can have lasting consequences for the outcome of future glaucoma surgery. The risk of failure for future glaucoma surgery is increased in those patients on longterm topical glaucoma therapy [10-13]. One potential reason for this is conjunctival inflammation linked to chronic topical ocular medication use. Significant increases in conjunctival fibroblasts, mast cells, lymphocytes and macrophages along with reduced numbers of epithelial goblet cells have been reported in patients receiving long-term topical glaucoma therapy. A risk factor for glaucoma surgery failure, postoperative fibrosis, may be more likely in eyes that have undergone long-term exposure to topical medications [14,15].
In addition to these cellular changes, a plethora of patient-centric adverse effects of topical ocular drugs have been well documented. Prominent among these are adverse changes to the ocular surface. A high percentage of glaucoma patients on preserved topical medications experience symptoms of ocular surface disease [16]. Overall, ocular surface disease is underdiagnosed and estimated to affect 40% to 59% of glaucoma patients globally [17]. Long-term use of these medications can cause discomfort, epithelial apoptosis, corneal surface impairment, conjunctival inflammation, dry eye, tear film instability, sub-conjunctival fibrosis, and meibomian gland dysfunction. The incidence of dry eye, increases with increasing number of medications in the regimen [18,19].
Individuals on multiple topical medications show higher Ocular Surface Disease Index (OSDI) scores, indicating worse ocular surface health, than those on one drop [20]. These scores tend to improve when patients are taken off drops or the number of drops per day is reduced [21]. Studies have demonstrated the negative impact of declining Ocular Surface Disease (OSD) from preserved topical medications on health-related Quality of Life (QoL) for glaucoma patients [22-24]. For example, Rossi et al., found that the more topical drops instilled per day, the lower the scores on the National Eye Institute-Visual Functioning Questionnaire (NEI-VFQ) 25 for vision-related quality of life [25]. The authors also reported that having more topical medications in a regime was associated with more severe symptom scores on the OSDI, which measures the effect of dry eye on vision-related QoL (with subscales on symptoms, function and triggers). This worsening of OSD can ultimately lead to reduced adherence to topical glaucoma medications. Conversely, having fewer topical drops in a therapeutic glaucoma regimen may equate to a better quality of life, particularly in areas related to ocular symptoms and performing daily activities [26].
Another issue associated with topical drops is their reliance on patient adherence, which is widely recognized to be low. Several factors for poor adherence have been identified by Tsai et al., [27,28]. These have been classified into patient, regional, provider, and situational/environmental categories. In terms of patient factors, individuals may lack the knowledge or skill to administer their drops and forgetting to take the drops is a common problem. The patient may lack motivation due to the silent nature of the disease or the belief that taking their medications is having no effect. Comorbidities can interfere with successful topical therapy, such as reduced visual acuity or arthritis, which can impair manual dexterity. Dry eye symptoms are also considered a predictor of non-adherence to a topical medication regimen [29,30].
Regional factors include the need to refill a prescription and its associated cost. The complexity of the regimen can constitute a daily burden to the patient. The required changes to a medication regimen as the disease progresses can be hard to incorporate into daily life. As was previously mentioned, side effects can have an adverse effect on ocular surface health and ultimately QoL. Provider factors for adherence include a lack of communication on the importance of consistent dosing to maintain control of intraocular pressure. Also, patients may experience dissatisfaction with their providers when they perceive little or no change to their visual acuity while having to deal with side effects of the medications and preservatives. Finally, situational and environmental factors can affect a patient’s daily life. A lack of accountability by the patient and/or a lack of support by the family or caregivers can decrease medication adherence [31]. Major life events, travel, competing activities, and changes in routine can interfere with or interrupt a daily routine of taking topical glaucoma medications. This patient population also typically has other comorbidities that may require multiple oral systemic medications, which may further challenge compliance with the treatment regimen.
For all of the above-elucidated reasons, adherence to topical medications is generally poor, and it tends to decrease with medication burden in both dosing frequency and number of bottles [32-35]. In addition, adherence worsens with increasing complexity of a topical medication regimen [36-38]. Poor adherence to ocular hypotensive medications can lead to more pronounced diurnal IOP fluctuations, which are associated with an increased risk of progressive loss of visual field [39]. Higher standard deviation of IOP, mean IOP, and maximum IOP are all associated with higher risks for glaucoma progression [40-43]. In addition, IOP elevations that can affect glaucoma progression are known to occur at night due to postural changes and/or trough medication concentrations [44-46]. Current first-line topical treatments may not sufficiently control these IOP fluctuations throughout the night [47]. In contrast, intracameral implants have been shown to control IOP fluctuations throughout the 24-hour diurnal period [48]. Minimizing diurnal fluctuation with continuous sustained IOP control may be more important over the long term for preventing disease progression than simple quantity-based reduction [49].
In addition to being associated with longer-term visual field progression, topical medications can have immediate dangers. Some topical medications also may be contraindicated in patients due to certain comorbidities or systemic medications. Agents such as beta-blockers are associated with rare serious systemic side effects such as arrhythmia, bradycardia, heart block, bronchospasm and worsening of underlying asthma or chronic obstructive pulmonary disease [50,51]. The Blue Mountains Eye Study (BMES), for example, found higher rates of mortality in glaucoma patients treated with topical timolol [52]. Fraunfelder et al., also reported over 30 deaths attributed to topical betablocker use [5]. Although not all studies have found this same effect, the risk of serious systemic side effects remains a valid concern [53-55].
Intracameral implants
In an effort to circumvent the risks and limitations of topical antihypertensive medications, several sustained release drug delivery alternatives have been introduced. This has been part of a broader shift away from a topical-medication-first paradigm to one using earlier proactive procedural intervention. These interventions may include laser trabeculoplasty, Minimally Invasive Glaucoma Surgery (MIGS), and sustained-release procedural pharmaceutical methods of delivering glaucoma medications to target tissues.
Among the sustained-release procedural pharmaceuticals for glaucoma, two intracameral implants have been approved by the U.S. Food and Drug Administration (FDA). These include the bimatoprost intracameral implant (Durysta®, approved in 2020; Allergan, Irvine, CA) and travoprost intracameral implant (iDose® TR, approved in 2023; Glaukos Corporation, Aliso Viejo, CA) [56-61]. These drug delivery systems were developed to provide targeted drug delivery to the desired sites by overcoming ocular surface barriers [61]. Both are approved for the reduction of IOP in patients with Open-Angle Glaucoma (OAG) and Ocular Hypertension (OHT).
Durysta efficacy: The bimatoprost intracameral implant is a biodegradable single-use sterile intracameral implant that contains 10 μg bimatoprost and is implanted directly into the anterior chamber of the eye. A prospective, 24-month, dose-ranging, paired-eye controlled, multinational, phase I/II clinical trial examined the IOP-lowering efficacy of the bimatoprost intracameral implant. Patients were randomized to receive either the bimatoprost implant (6 μg, 10 μg, 15 μg or 20 μg; the 10 μg implant was ultimately chosen for commercialization) intracamerally in the study eye or topical bimatoprost 0.03% once daily in the fellow eye. Mean IOP reductions for the 6 μg, 10 μg, 15 μg and 20 μg groups were 7.5 mmHg, 7.3 mmHg and 8.9 mmHg, respectively, compared to 8.2 mmHg for the topical bimatoprost group [62,63]. At 6, 12 and 24 months, 68%, 40% and 28% of study eyes, respectively, did not require rescue medications or retreatment. Overall, the bimatoprost implant group achieved IOP-lowering effects comparable to those of topical bimatoprost.
The efficacy of bimatoprost implant has been evaluated in two phase III multicenter, randomized, parallel-group, active-controlled, 20-month (including an 8-month extended follow-up) studies of bimatoprost implant compared to twice daily topical timolol 0.5% drops, in patients with OAG or OHT (ARTEMIS-1 and 2; NCT02247804 and NCT02250651, respectively) [64-67]. Patients underwent washout periods of up to 42 days, depending on the topical medications they were taking at the time of screening. In the Artemis-1 and Artemis-2 studies, eyes receiving either the implant or topical timolol experienced an IOP reduction of approximately 5-8 mmHg in patients with a mean baseline IOP of 24.5 mmHg. The bimatoprost implant achieved non-inferiority with topical timolol through week 12 (the primary endpoint) (Figures 1 and 2).
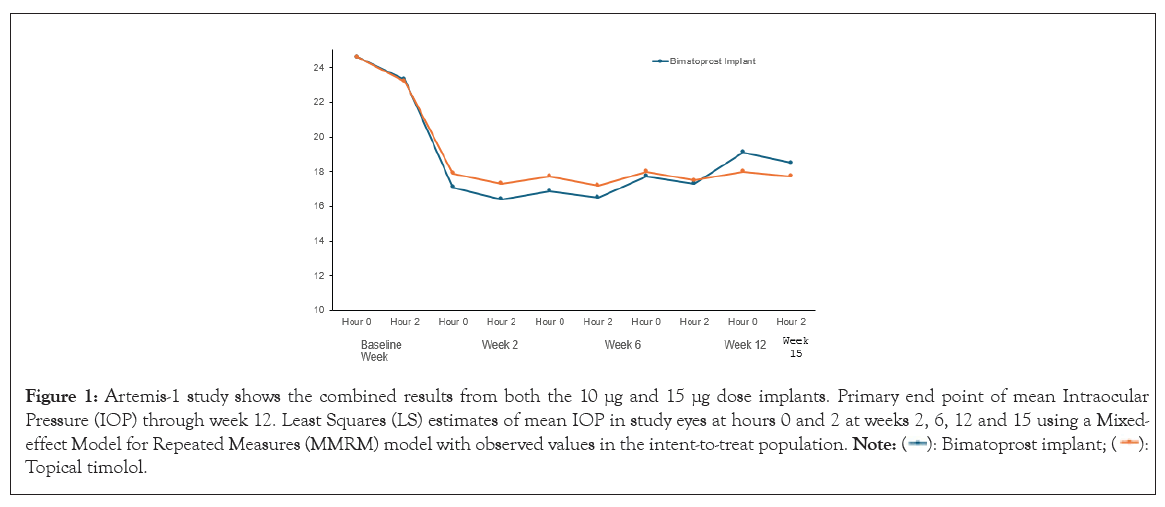
Figure 1: Artemis-1 study shows the combined results from both the 10 μg and 15 μg dose implants. Primary end point of mean Intraocular
Pressure (IOP) through week 12. Least Squares (LS) estimates of mean IOP in study eyes at hours 0 and 2 at weeks 2, 6, 12 and 15 using a Mixed-effect
Model for Repeated Measures (MMRM) model with observed values in the intent-to-treat population.  Topical timolol.
Topical timolol.
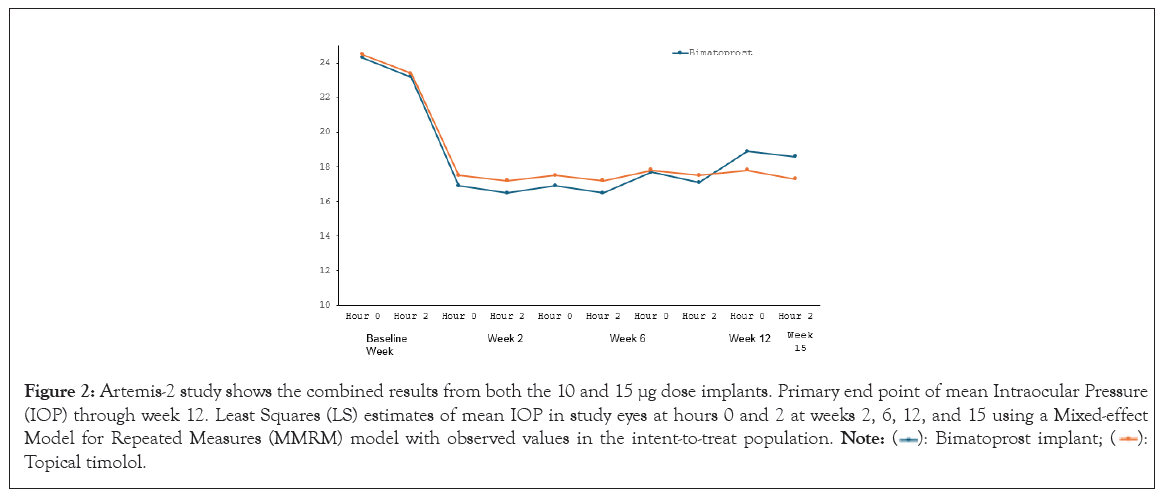
Figure 2: Artemis-2 study shows the combined results from both the 10 and 15 μg dose implants. Primary end point of mean Intraocular Pressure
(IOP) through week 12. Least Squares (LS) estimates of mean IOP in study eyes at hours 0 and 2 at weeks 2, 6, 12, and 15 using a Mixed-effect
Model for Repeated Measures (MMRM) model with observed values in the intent-to-treat population.  Topical timolol.
Topical timolol.
Durysta safety: The most common ocular adverse reaction observed in the two phase III clinical trials with the bimatoprost intracameral implant was conjunctival hyperemia, which was reported in 27% of patients. Other common ocular adverse reactions reported in 5%-10% of patients were foreign body sensation, eye pain, photophobia, conjunctival hemorrhage, dry eye, eye irritation, IOP increased, corneal endothelial cell loss, vision blurred and iritis. Ocular adverse reactions occurring in 1%-5% of patients were anterior chamber cell, increased lacrimation, corneal edema, aqueous humor leakage, iris adhesions, ocular discomfort, corneal touch, iris hyperpigmentation, anterior chamber flare, anterior chamber inflammation and macular edema. Many of the Adverse Events (AEs) reported in the study eyes occurred within 2 days after implantation and were likely associated with the implant procedure. AEs were typically mild in severity and quick to resolve.
Safety assessments with the bimatoprost implant included endothelial cell density by specular microscopy. In the phase III study, mean corneal endothelial cell density over time was measured in study eyes treated with one to three administrations of the bimatoprost implant (10 μg or 15 μg) and fellow eyes treated with twice daily topical timolol maleate 0.5%. Adverse events that most often lead to early study exit for the bimatoprost implant groups included endothelial cell loss. Corneal endothelial cell loss was more frequent in both bimatoprost implant groups and was the most frequent serious ocular adverse event in these groups. Endothelial cell density showed a time-dependent and dose-dependent decrease in both bimatoprost implant groups, with endothelial cell loss being more common after repeat treatment and at the higher 15 μg dose (which was ultimately not commercialized). In the 10 mg and 15 mg bimatoprost implant groups, respectively, a ≥ 20% decrease in Endothelial Cell Density (ECD) vs. baseline was seen in 0% and 2.8% of study eyes at 12 weeks after initial administration (week 12), in 2.3% and 6.3% of study eyes at 12 weeks after the second administration (week 28), in 4.1% and 12.3% of study eyes at 12 weeks after the third administration (week 44), and in 10.2% and 21.8% of study eyes at the month 20 or the last study visit before exit. This was compared to the timolol group, in which a ≥ 20% ECD decrease vs. baseline was seen in no study eyes at 12 weeks after the first, second or third administration and in 0.5% (1/197) of study eyes at the month 20 or last study visit before exit. The implant is recommended for a single implantation with no retreatment.
Pharmacokinetic evaluations showed systemic bimatoprost concentrations were below the limit of detection (0.001 ng/mL) in most (92%) of the patients following bimatoprost intracameral implant administration. Bimatoprost implant was generally well received with 82.9% of patients responding that they would be very or extremely likely to have another implant procedure and 88.6% indicating that they would recommend the procedure to someone else with glaucoma or ocular hypertension.
iDose Travoprost intracameral implant (TR) efficacy: The travoprost intracameral implant is designed to provide continuous sustained IOP-lowering therapy, thereby reducing patient treatment burden and improving adherence. This biocompatible implant is implanted directly through the trabecular meshwork, compressing the tissue until the implant anchor securely penetrates the sclera through the back wall of Schlemm’s canal (Figure 3).
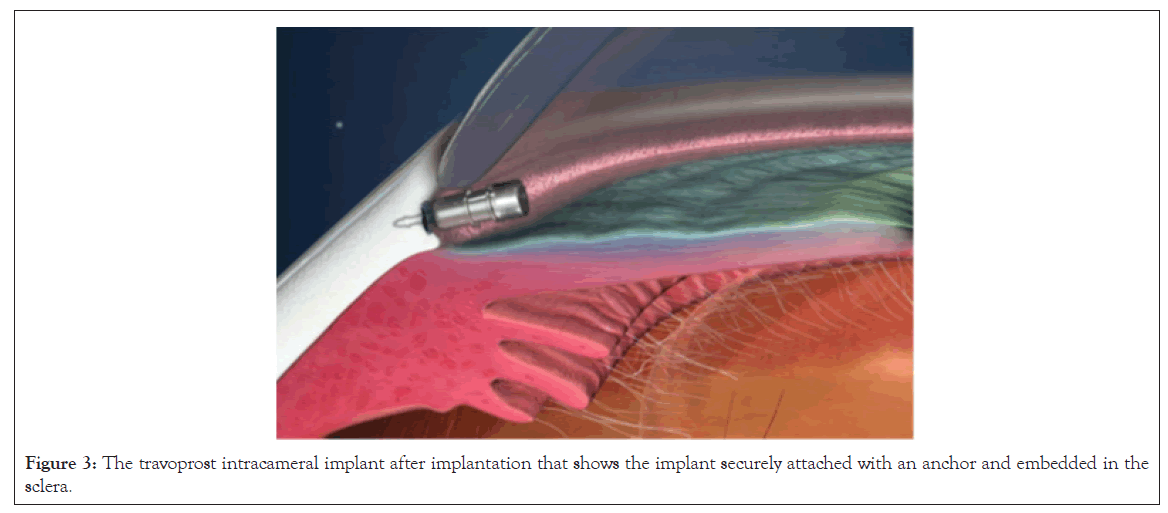
Figure 3: The travoprost intracameral implant after implantation that shows the implant securely attached with an anchor and embedded in the sclera.
A randomized, double-masked, multicenter, phase IIb trial was conducted to evaluate the long-term safety and efficacy of the extended-release continuous drug delivery system travoprost intraocular implant (NCT02754596) in patients with open angle glaucoma or ocular hypertension [68,69]. Patients on topical glaucoma medications at screening underwent washouts of up to 8 weeks depending on the type of medication they were taking at screening. The treatment groups included a Fast-Eluting implant (FE implant, n=51) and twice daily Bis In Die (BID) placebo eye drops, a Slow Eluting implant (SE implant, n=54) and BID placebo eye drops, and a sham procedure and BID timolol 0.5% (n=49). The SE implant was ultimately chosen for commercialization.
IOP measurements were made preoperatively and at day 10, week 6, months 3, 6, 9, 12, 18, 24, 30 and 36. Mean reductions in IOP were observed at all visits for all treatment groups and maintained out to month 36. In the subgroup of SE and FE implant patients who were well controlled on the same or fewer topical IOP-lowering medications reductions from screening, significant IOP reductions were achieved at 12, 24 and 36 months versus baseline (Figure 4).
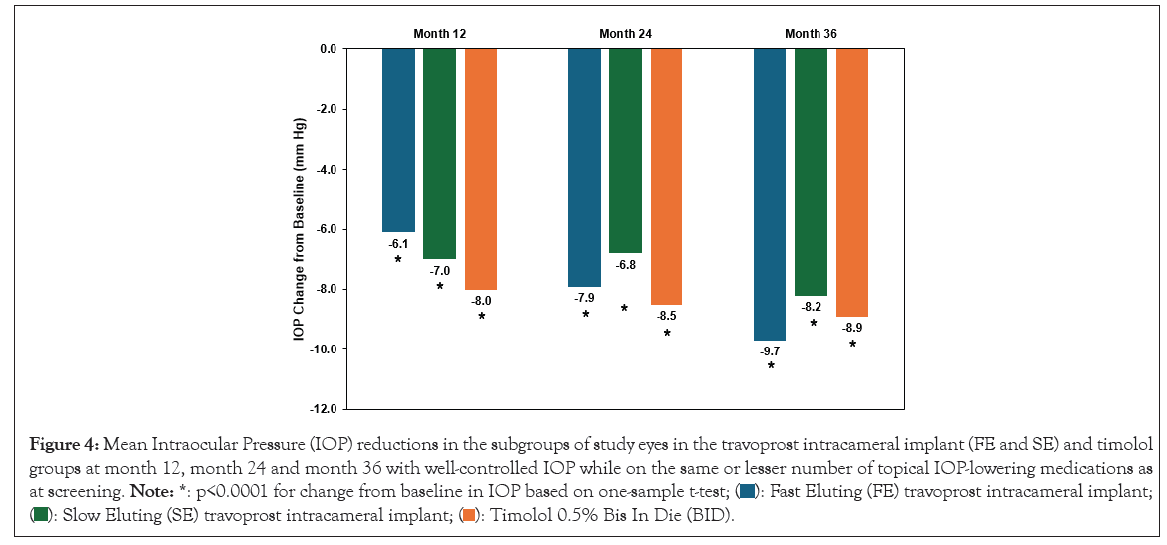
Figure 4: Mean Intraocular Pressure (IOP) reductions in the subgroups of study eyes in the travoprost intracameral implant (FE and SE) and timolol
groups at month 12, month 24 and month 36 with well-controlled IOP while on the same or lesser number of topical IOP-lowering medications as
at screening. Note: *: p<0.0001 for change from baseline in IOP based on one-sample t-test;  travoprost intracameral implant;
travoprost intracameral implant; 
The percentage of responders (those with well-controlled IOP who did not require additional topical IOP-lowering medications per the protocol-specified criteria) was significantly higher in the implant groups at 12, 24 and 36 months compared to the topical timolol group. Together these data show robust and sustainable IOP and medication reductions through month 36 with travoprost intracameral implant administration (Figure 5).
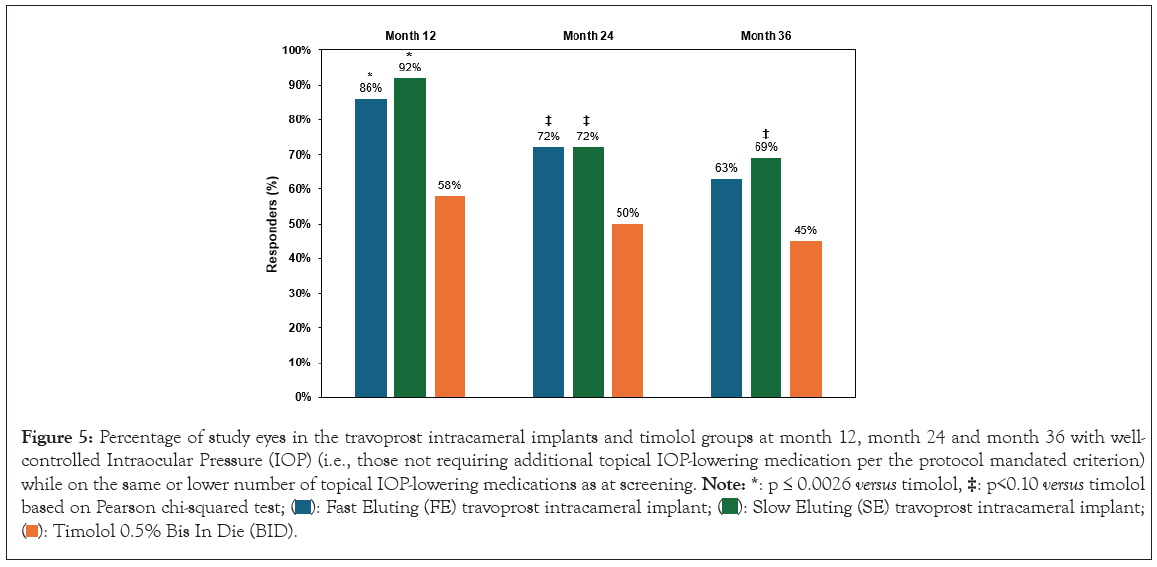
Figure 5: Percentage of study eyes in the travoprost intracameral implants and timolol groups at month 12, month 24 and month 36 with well-controlled
Intraocular Pressure (IOP) (i.e., those not requiring additional topical IOP-lowering medication per the protocol mandated criterion)
while on the same or lower number of topical IOP-lowering medications as at screening. Note: *: p ≤ 0.0026 versus timolol,  based on Pearson chi-squared test;
based on Pearson chi-squared test; 
Following the favorable phase II results, two identically-designed phase III, parallel-group, double-masked, randomized, prospective, sham-controlled trials were completed to compare both FE and SE travoprost intracameral implants to topical timolol (0.5%) BID for patients with OAG or OHT (GC-010, NCT03519386 and GC-012, NCT03868124; N=1150) [70]. In both of these trials, the implant group patients achieved the pre-specified primary efficacy endpoint of non-inferiority to topical timolol through 3 months. In the first 3 months following travoprost implant administration, iDose TR patients experienced an IOP change from baseline of -6.6 mmHg to -8.4 mmHg in the study eye from a mean baseline IOP of 24 mmHg (Figures 6 and 7).
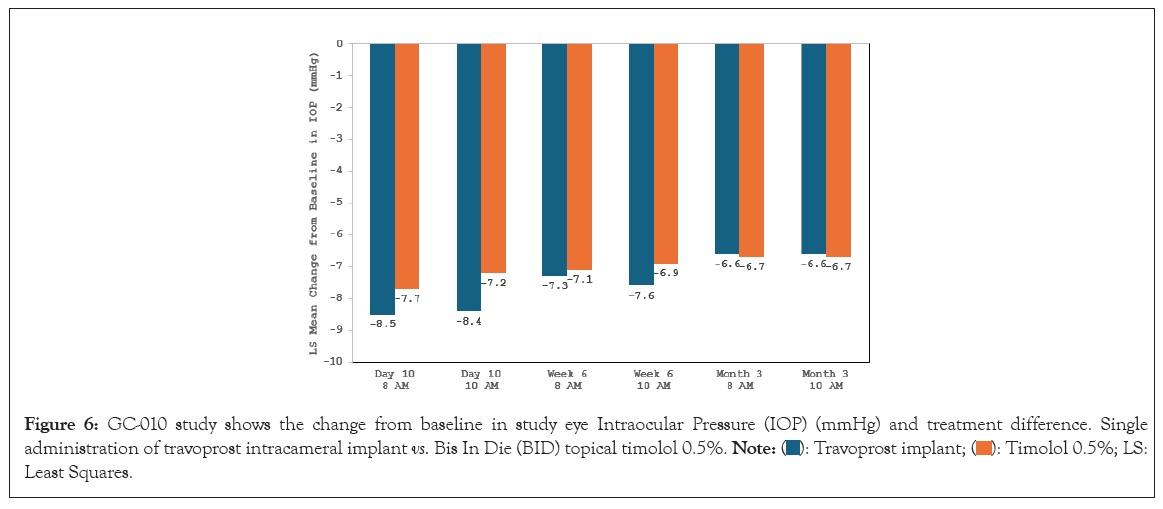
Figure 6: GC-010 study shows the change from baseline in study eye Intraocular Pressure (IOP) (mmHg) and treatment difference. Single
administration of travoprost intracameral implant vs. Bis In Die (BID) topical timolol 0.5%.  Least Squares.
Least Squares.
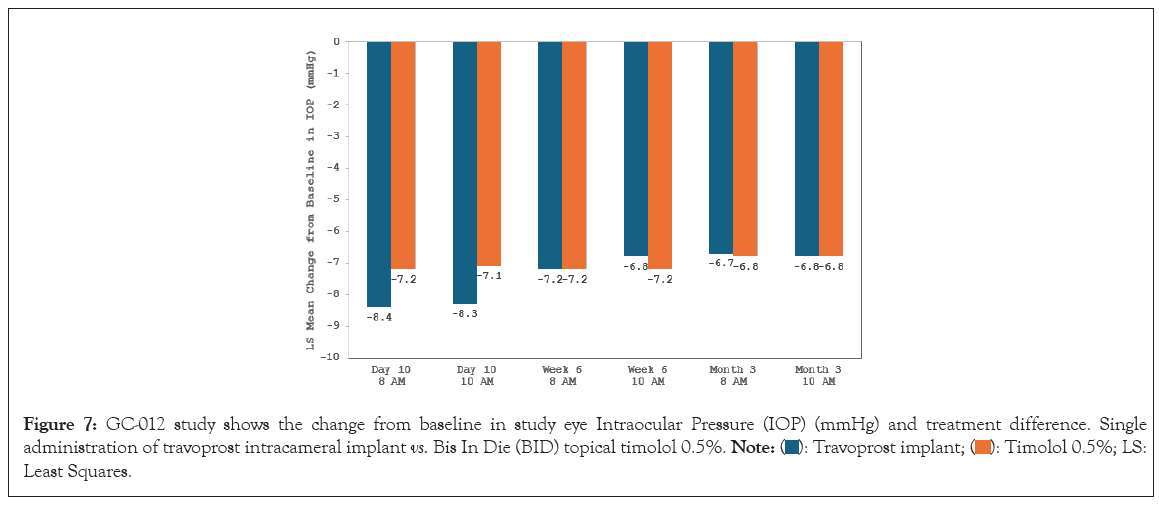
Figure 7: GC-012 study shows the change from baseline in study eye Intraocular Pressure (IOP) (mmHg) and treatment difference. Single
administration of travoprost intracameral implant vs. Bis In Die (BID) topical timolol 0.5%.  Least Squares.
Least Squares.
Subsequently, the travoprost intracameral implant demonstrated noninferiority to BID timolol over the next 12 months, a key secondary endpoint. The fast and slow eluting implants exhibited similar IOP-lowering profiles over the 12-month study period. At the beginning of these two trials, 23% of patients overall were on 2 or more ocular hypotensive medications. At 12 months in the two studies, 81% of the iDose TR patients were completely free of topical IOP-lowering topical medications. For patients who were on glaucoma medication at screening, a significantly greater percentage in the SE implant group (83.5%) and in the FE implant group (78.7%) compared with the timolol group (23.9%) were on fewer topical glaucoma medications at month 12 compared to baseline (SE implant versus timolol, p<0.0001; FE implant versus timolol, p<0.0001; chi-square test) (Figure 8).
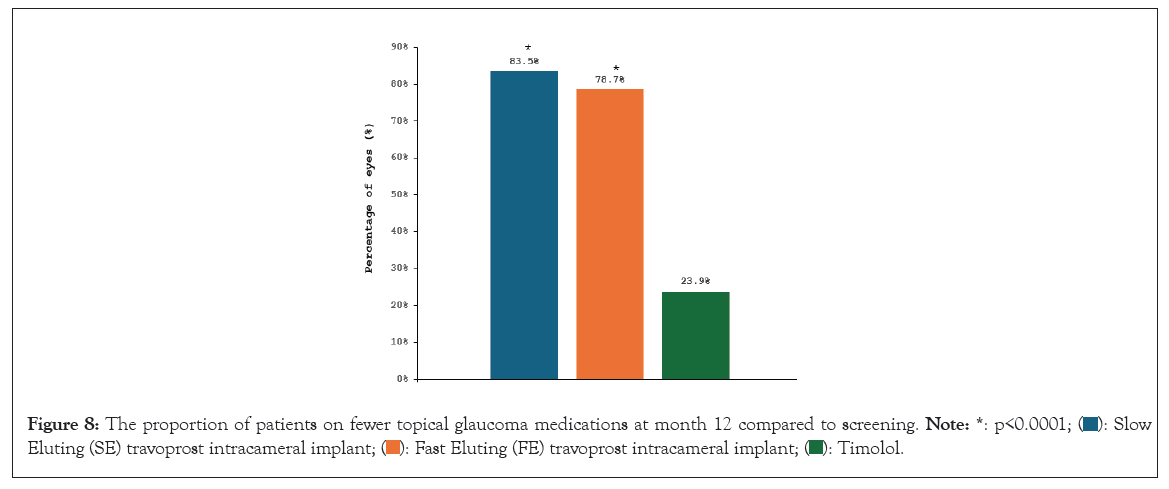
Figure 8: The proportion of patients on fewer topical glaucoma medications at month 12 compared to screening.  Eluting (SE) travoprost intracameral implant;
Eluting (SE) travoprost intracameral implant; 
iDose Travoprost intracameral implant (TR) safety: Adverse event rates for the travoprost intracameral implant originated from the aforementioned three randomized, double-masked clinical trials (one phase II and two phase III trials) including 868 implant patients with OAG or OHT who were followed for one year. The most commonly reported ocular adverse reactions, in 2% to 6% of patients, were increased IOP, iritis, dry eye, visual field defects, eye pain, ocular hyperemia and reduced visual acuity. Ocular adverse reactions reported in less than 2% of patients included conjunctival hemorrhage, photophobia, punctate keratitis, blepharitis, eye irritation, corneal abrasion, implant dislocation, vitreous detachment and foreign body sensation in eyes.
Importantly, mean corneal endothelial cell counts were stable for all three treatment groups. At month 36, no statistically significant differences in change from baseline in endothelial cell counts were detected between the implant groups and the timolol group (p>0.1730). In addition, no clinically meaningful changes from baseline occurred in central corneal thickness, visual field Mean Deviation (MD) or Best-Corrected Visual Acuity (BCVA), for any treatment group.
Most patients (slow-eluting implant group, 90.3% and fast eluting implant group, 91.0%) receiving the travoprost intracameral implant had normal or trace conjunctival hyperemia scores at baseline after the protocol-prescribed washout period. Following the procedure, slightly fewer patients had normal or trace levels of hyperemia. During the course of the study, mean conjunctival hyperemia scores were below 0.5 (out of 3) from baseline to month 12. Most of the patients had returned to baseline hyperemia levels by the 12-month assessment. No patient in any treatment group had an increase in iris pigmentation or experienced periorbital fat atrophy during the 12-month study period. The travoprost intracameral implant was generally well received, with only 5 patients (<1%) discontinuing the study prior to month 12 due to treatment-emergent adverse events [70].
In addition, a pharmacokinetic study of 105 patients found the plasma concentrations of travoprost free acid were below the limit of detection (10 pg/mL) in all patients at day 10, week, 12 and month 12 following travoprost intracameral implant administration.
Topical ocular hypotensive medications have a record of IOP-lowering efficacy in clinical trials. These medications target the eye directly and minimize systemic side effects compared to oral medications. Different classes of IOP lowering agents have distinct methods of action that can be tailored to each individual patient. Some agents such as prostaglandins only need to be administered once daily. Furthermore, IOP reduction from topical agents is relatively quick, providing the opportunity for immediate control without the systemic considerations of oral agents. These are some of the reasons why topical IOP lowering agents are commonly employed as first-line treatments for glaucoma.
However, topical medications have associated caveats which affect their long-term success. For example, the preservatives in many topical medications can be detrimental to the ocular surface with long-term administration [29]. This can result in dry eye, tear film instability, corneal and conjunctival inflammation, long-term ocular surface damage, and patient discomfort. Both side effects and medication non-adherence are known to worsen with more medications in a regimen, which is consequential given that approximately half of glaucoma patients need multiple topical medications to control IOP adequately. Topical therapy can have a negative effect on later glaucoma surgical success [10,11]. Reduced health-related quality of life also can be a problem for glaucoma patients [2,17].
Long-term topical medication therapy represents a considerable healthcare burden, both to patients and their caregivers. And even in patients being followed and treated for glaucoma, visual decline can occur. For example, a population-based study in Olmsted County, Minnesota showed that the probability of glaucoma-related blindness within 20 years was 13.5%-25.8% [71]. Ultimately, those who experience a loss of visual function may struggle to perform daily tasks of living.
Additionally, one of the chief obstacles for treatment success with topical glaucoma medications is the considerable difficulty in adhering to prescribed therapeutic regimens. The asymptomatic, slowly progressive nature of the disease can present a challenge as patients often cannot tell any subjective effect of continued treatment [72]. Thus, patients are faced with the immediate downsides of topical treatments compared to the distant risks of vision loss, a tradeoff which may be particularly burdensome for patients at earlier stages of the disease who have full visual function. Suboptimal compliance with resulting inadequate IOP control can put patients at risk for progressive visual field loss [42]. This issue is not unique to glaucoma. Poor adherence to medications in general is estimated to cost up to 10% of total healthcare spending [73]. Disease severity also has substantial financial ramifications for patients and health systems. A study by Lee et al., showed significantly increased expenditure with advancing severity of glaucoma, with the cost of patients with impaired vision due to glaucoma estimated at over 2 billion U.S. dollars per year [74].
Intracameral implants have been developed to address many of the shortcomings associated with topical ocular medications. This method of delivery is designed to overcome the ocular surface barriers to provide a therapeutic concentration at the target sites in a continuous 24/7 manner [61]. The bimatoprost and travoprost implants have been shown to reduce IOP and topical medication burden [75], resulting in reduction or elimination of reliance on preserved topical medications [68,70], with data suggesting months-long duration for the bimatoprost implant [66] or years-long duration for the travoprost implant [68]. This reduces the overall treatment burden, improves adherence [62], and provides continuous sustained release of medication to target tissues.
Intracameral implants have demonstrated favorable adverse effect profiles compared to topical medications. Patients may benefit from an enhanced QoL due to this reduction in treatment burden [26]. The implants are sterile/single-use drug delivery systems which deliver ocular hypotensive medications without the need for preservatives [56,59], avoiding deleterious effect to the ocular tissues. Intracameral delivery bypasses the ocular surface to spare the cornea and conjunctiva, effectively delivering drug directly to the target tissues-the trabecular meshwork, Schlemm’s canal and sclera via the uveoscleral outflow route. Systemic exposure is also minimized [61], and fewer diurnal IOP fluctuations than what is commonly seen with topical medications are expected with intracameral therapy.
With any given glaucoma therapy, it is important to remember that no treatment is a panacea, and different treatment(s) may be appropriate for different patients. Ophthalmologists must consider the pros and cons of the options available. For example, topical medications are relatively quick-acting and easy to prescribe, they require no intraocular procedures, and they are often expected by patients; however, they are associated with adverse ocular surface effects, poor compliance, systemic reactions (including fatalities), greater IOP fluctuations, more Visual Field (VF) progression, and higher risk of future surgical failure. Intracameral implants provide sustained medication directly to target tissues, preserve the health of the ocular surface, and eliminate the challenge of medication non-compliance; however, they necessitate an intraocular procedure (with associated risks, albeit small); they must be implanted in the office, procedure room or operating room; in some cases (Durysta) they can be associated with corneal endothelial cell damage; and they have months-long (Durysta) or years-long (iDose TR) duration of effect. An interventional approach to glaucoma management does not ignore these pros and cons, but rather takes them into thoughtful consideration of what’s best for the patient. Given the evidence available, intracameral procedural pharmaceuticals may be a preferable alternative to long-term chronic topical drop therapy. Indeed, it may be time to rethink the former topical-medications-first treatment paradigm and recognize the evidence showing intracameral implants to be a valuable addition to the ophthalmologist’s treatment armamentarium for glaucoma and ocular hypertension.
Julie Crider, PhD and Dana M. Hornbeak, MD, MPH are acknowledged for medical writing contributions.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Kamat S, Baudouin C, Shah M, Radcliffe N (2024) Long-Term Chronic Drop Therapy vs. Intracameral Procedural Pharmaceuticals for Glaucoma: What Does the Evidence Support? J Clin Exp Ophthalmol. 15:986.
Received: 02-Aug-2024, Manuscript No. JCEO-24-33322; Editor assigned: 05-Aug-2024, Pre QC No. JCEO-24-33322 (PQ); Reviewed: 19-Aug-2024, QC No. JCEO-24-33322; Revised: 26-Aug-2024, Manuscript No. JCEO-24-33322 (R); Published: 03-Sep-2024 , DOI: 10.35248/2155-9570.24.15.986
Copyright: © 2024 Kamat S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.