Clinical & Experimental Cardiology
Open Access
ISSN: 2155-9880
ISSN: 2155-9880
Review - (2022)Volume 13, Issue 11
Introduction: The treatment of peripartum cardiomyopathy follows the main principles of the treatment of heart failure with reduced ejection fraction. To date, the adoption of bromocriptine as a legitimate option in the PPCM treatement is widely variable across the world. The main objective of this work was to evaluate the efficacy of bromocriptine in improving left ventricular function in addition to conventional treatment of peripartum cardiomyopathy. Methodology:This was a longitudinal, prospective quasi-experimental study conducted in patients followed at the cardiology clinic of Aristide Le Dantec Hospital for peripartum cardiomyopathy from January 1, 2017 to January 01, 2021, with a total duration of 48 months. We made a consecutive and exhaustive recruitment of all patients admitted during the study period. This study received the approval of the ethics committee of the Cheikh Anta Diop University of Dakar. A Kaplan Meier survival curve was produced to compare the evolution of LVEF in patients on Bromocriptine versus patients on conventional treatment. Results: During the recruitment period, 540 patients were hospitalized, including 320 women. Taking into account the inclusion criteria, 55 patients were selected. The rate of hospitalization in the cardiology department for peripartum cardiomyopathy was 5.8%. The average age was 30.5 ± 6.7 years with extremes of 18 years and 42 years. Left ventricular systolic dysfunction was found in all our patients with an average systolic ejection fraction calculated by Simpson biplane of 27.9% ± 8.69%. Eleven (11) patients were in addition to treatment for heart failure with bromocriptine versus 44 with treatment for heart failure alone. After 6 months of follow-up, left ventricular systolic function in patients on bromocriptine had increased from 28.45% to 53.6%; Whereas that of the standard treatment group had increased from 28.27% to 42.1% (p=0.007). Patients on Bromocriptine recovered twice as quickly at 6 months and had better survival (p=0.036) compared to those on conventional treatment for heart failure only. Conclusion: Peripartum cardiomyopathy is a severe heart condition that worsens maternal mortality in sub-Saharan Africa. Bromocriptine improves the short-term and long-term prognosis of these patients. Large cohort clinical trials should be conducted in sub-Saharan Africa to better prove its effectiveness where this condition is more common.
Bromocriptine; Cardiomyopathy; Peripartum
Peripartum Cardiomyopathy (PPCM) is left ventricular systolic dysfunction (LVEF less than 45%) [1], without identifiable aetiology, occurring at the end of pregnancy or during the months following childbirth, responsible for a clinical heart failure. It is a rare pathology in Western countries, frequent in sub-Saharan Africa. The incidence varies according to ethnicity, it is higher in patients of African origin, rare in Europe [2,3]. The evolution of PPMC is highly variable, with nearly one-third to one-half of patients improving their left ventricular function within the first six months postpartum. However, PPCM can be potentially fatal because it leads to serious complications (severe heart failure, cardiogenic shock, severe ventricular arrhythmias and thromboembolic events). The mortality rate varies from 0 to 28% according to published data. [4,5]. With the discovery of the oxidative stress, cathepsin D, prolactin 16 kDa cascade, a new hypothesis emerges with a possible treatment with bromocriptine which is a prolactin inhibitor. Bromocriptine has thus been suggested as a new treatment for PPCM. It inhibits the secretion of prolactin, thus preventing the formation of the N-terminal fragment of prolactin of 16 kDA [6]. In Africa, Sliwa [7] had reported in a princeps study the efficacy of bromocriptine in a small cohort. On the other hand, the results of the IPAC study [8] in subjects for whom bromocriptine was not used seem to be comparable to those of patients with CMPP from German researchers who received bromocriptine in addition to the standard treatment based on proofs. More recently, Hilfiker-Kleiner et al. had found an improvement in Left Ventricular Ejection Fraction (LVEF) at 6 months by comparing a short and long course of bromocriptine (n=63) [9]. This trial was informative for the favorable results and relative safety of bromocriptine in PPCM, but due to the lack of a bromocriptine-free control group, the efficacy conundrum has not been fully resolved [9]. The body of evidence from clinical trials and observational studies evaluating the effect of bromocriptine on outcome remains sparse. Furthermore, the abrupt loss of breast milk following the use of bromocriptine has an immediate negative effect on infant nutrition, and particularly in our situations where formula milk is neither affordable nor available. So despite the results reported in the literature, bromocriptine is still under used because there is still the problem of broad validation. The main objective of this work was to evaluate the efficacy of bromocriptine in improving left ventricular function in addition to conventional treatment of peripartum cardiomyopathy.
This work was carried out at the cardiology clinic of the CHU Aristide Le Dantec in Dakar from January 01, 2017 to January 01, 2021, i.e. a total duration of 48 months. This study received the approval of the ethics committee of the Cheikh Anta Diop University of Dakar (CER/UCADI AD (MSN/012/2020). It is a clinical, longitudinal and prospective quasi-experimental study conducted in patients admitted to the cardiology clinic of Aristide Le Dantec Hospital for peripartum cardiomyopathy. We conducted a consecutive and exhaustive recruitment of all patients admitted to the cardiology department of Aristide la Dantec Hospital. We included all patients hospitalized or followed in the service in whom a diagnosis of CMPP according to the definition criteria of the working group of the European Society of Cardiology on CMPP [1].
Not included were any patients with an echocardiographic appearance not responding to the diagnosis, or having another known significant heart disease or any other cause of heart failure or any patient refusing to participate in the study. We analyzed epidemiological, clinical and paraclinical data such as transthoracic echocardiography with the parameters of the diameters of the heart chambers, the kinetics and the systolic function of the left ventricle; the existence of thrombus or spontaneous intra-cavity contrast.We reviewed the patients after hospitalization to assess their clinical condition, and follow-up ultrasounds were performed every 3 months in the echocardiography laboratory of the Aristide le Dantec university hospital center. This echocardiography laboratory is headed by a full professor of cardiology with a degree in echocardiography with more than 10 years' experience and his assistant with a degree in echocardiography with more than 10 years' experience.
We evaluated the recovery rate for this we considered an LVEF greater than 50% as a criterion for complete recovery and the absence of remission if the LVEF remained below 45% or if a death was noted or a complication. For this study, we used the Vivid E9 echocardiography device equipped with an adult matrix probe with excellent spatial and temporal resolution. The primary endpoint was recovery of left ventricular ejection fraction (LVEF ≥ 50%). The secondary endpoints were an unfavorable outcome such as the occurrence of heart failure, or thromboembolic events, or the persistence of left ventricular dysfunction or death.
Statistical aspects
We calculated the sample size with the Student's t test for a difference in mean with two independent samples based on the princeps study by Sliwa [7]. Thus, to detect a 20% improvement in LVEF (i.e. 56% LVEF in patients in the bromocriptine group versus 36% in the group without bromocriptine), a number of 22 patients was therefore necessary with 11 on bromocriptine in more than conventional heart failure therapy and 11 on conventional heart failure therapy. With this sample size, the power is 95% with a two-tailed test and a confidence level of 1%. Taking into account possible loss of sight and non-adherence to the protocol, we will recruit 5 more patients in each arm, for a total number of 16 patients per arm. To select patients comparable to the group of patients on bromocriptine, we matched the variables likely to interfere with the prognosis or the outcome of our patients. These were the following variables: age, twinning, multiparity, pregnant hypertension, NYHA III/IV stage and DTDVG ≥ 55 mm and LVEF<35%. All the data collected were entered into the Excel software and the analysis was carried out by the SPSS software in its version 26 and also by Excel.
The description of the qualitative variables was made using percentages and those of the quantitative variables using means and standard deviations. The different frequencies were compared using the Chi² test and the Student's t test for the quantitative variables according to the normal law. Where appropriate, non-parametric tests were used. A value of p<0.05 was considered statistically significant. To identify the independent predictors of poor prognosis, we first performed a binary logistic regression to find the significant variables and then a multivariate logistic regression analysis was performed to highlight the independent variables of poor prognosis.
All values were expressed with Odds Ratio (OR) and 95% Confidence Intervals (CI). A p value <0.05 and a 95% CI not containing 1 was considered statistically significant. A Kaplan Meier survival curve was produced to compare the evolution of LVEF in patients on Bromocriptine versus patients on conventional treatment.
Epidemiological data
During the period of our study, 540 patients were hospitalized, including 320 women. Taking into account the inclusion criteria, 55 patients were selected. The rate of hospitalization in the cardiology department for peripartum cardiomyopathy was 5.8%.
Clinical characteristics of patients at inclusion
The average age was 30.5 ± 6.7 years with extremes of 18 years and 42 years. More than half of the patients, 60%, were under 30 years old as shown. The clinical characteristics of the population are presented in Table 1.
| Clinical features | Number | Percentage (%) | ||||
|---|---|---|---|---|---|---|
| Age | <30 years old | 33 | 60 | |||
| >30 years old | 22 | 40 | ||||
| Geographic origin | Urban | 24 | 43.7 | |||
| Rural | 31 | 56.3 | ||||
| Socio-economic level | ||||||
| Low | 78.2 | 43 | ||||
| Medium | 18.2 | 10 | ||||
| Raised | 3.6 | 2 | ||||
| Parity | ||||||
| Primiparous | 15 | 27.2 | ||||
| Multiparaity | 40 | 72.8 | ||||
| Twin pregnancy | 11 | 20 | ||||
| Pregnancy hypertension | 15 | 27.2 | ||||
| History of CMPP | 4 | 7.27 | ||||
| NYHA | Stage III | 5 | 9 | |||
| Stage IV | 50 | 91 | ||||
| Cardiovascular collaps | 3 | 5.4 | ||||
| Heart failure | 55 | 100 | ||||
| Moderate anemia (8 g-10 g) | 12 | 26.1 | ||||
| Radiological cardiomegaly | 26 | 47.2 | ||||
| Left ventricular hypertrophy (at ECG) | 27 | 49.1 | ||||
Table 1: Clinical characteristics of patients at inclusion.
Doppler echocardiography
It noted dilatation of the left ventricle in 43 patients (78.2%). The mean end diastolic diameter of the left ventricle (LVTD) was 59 ± 6.3 mm with extremes of 46 mm and 77 mm. Dilatation of the left atrium was noted in 52 patients. Left ventricular systolic dysfunction was found in all our patients with an average systolic ejection fraction calculated by Simpson biplane of 27.9 ± 8.69%. Five (5) patients, i.e. 9.1%, had a moderate alteration in the systolic function of the left ventricle and a severely impaired systolic function was found in 50 patients, (90.9%). Five (5) patients (9.1%) presented a left intraventricular thrombus. Spontaneous contrast was noted in 7 patients, (12.7%). A one case of moderate pericardial effusion was noted in our study. A moderate functional mitral regurgitation was observed in 13 patients or 23.6% of cases, it was associated with a tricuspid regurgitation in 11 cases or 20%. In our study, four patients had a history of PPCM.
Treatment
A sodium-restricted diet was instituted in all patients. Diuretics were used in all cases, only loop diuretics and anti-aldosterones were used in the acute phase. ACE inhibitors were prescribed in 52 cases in the acute phase, digitalis were prescribed in 4 patients. Forty-six (46) patients or 83.6% of the study population had a beta-blocker in their discharge prescription. All patients had received anticoagulant treatment with Anti-Vitamin K (AVK). Bromocriptine was prescribed in 11 patients. It was stopped after 12 days of treatment in a patient who presented with a thromboembolic complication such as pulmonary embolism. None of the patients had received immunosuppressive treatment or interventional treatment. The adjuvant treatment consisted of contraception which was effective in 50 patients, (91%). Iron supplementation in 10 patients. Non-specific antibiotic therapy in 7 patients.
Evolution
The average length of hospitalization was 8 ± 5.02 days with extremes of 4 and 33 days. The hospital evolution was favourable in 45 patients or 81.8% of cases. In hospitalization, 19 patients had complications, (34.5%). In-hospital mortality was 3.6%.This table below summarizes the complications we observed in our series (Table 2).
| Complications | Number | Percentage (%) |
|---|---|---|
| Cardiogenic shock | 3 | 5.5 |
| Refractory heart failure | 2 | 3.6 |
| Thrombi and intraventricular contrast | 12 | 21.8 |
| Pulmonary embolism | 1 | 1.8 |
| Hospital mortality | 2 | 3.6 |
Table 2: The complications noted in the population.
At inclusion, the two treatment groups, bromocriptine versus no bromocriptine, were comparable on the main variables factors of poor prognosis of CMPP. Table 3, shows the clinical characteristics of each group according to these factors.
| Baseline clinical characteristics | Bromocriptine (n=11) | TTT conventionnel (N=44) | p |
|---|---|---|---|
| Low socio-economic level | 8 (72.7%) | 42 (95.4%) | 0.05 |
| Multipara | 9 (81.8%) | 31 (70.4%) | 0.032 |
| Twin Pregnancy | 5 (45.4%) | 6 (13.6%) | 0.706 |
| Pregnancy hypertension | 2 (18.18%) | 13 (29.5%) | 0.706 |
| Heart failure | 8 (72.7%) | 42 (95.4%) | 0.05 |
| Average LVEF | 28.45% | 28.27% | 0.946 |
Table 3: Clinical characteristics of each group according to poor prognostic factors for PPCM at inclusion.
After 6 months of follow-up, left ventricular systolic function in patients on bromocriptine had increased from 28.45% to 53.6%; While that of the standard treatment group had increased from 28.27% to 42.1%. This difference was statistically significant (p=0.007) (Figure 1).
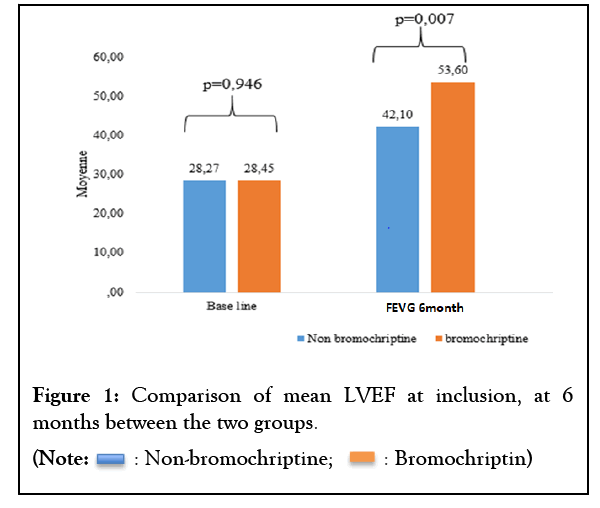
Figure 1: Comparison of mean LVEF at inclusion, at 6 months between the two groups. 
In this cohort, the patients on bromocriptine had a remission rate at 6 months that was twice as high compared to the other patients not treated with bromocriptine (Table 4).
| Bromocriptine/Status remission | Yes | No | Total |
|---|---|---|---|
| Remission | 7 | 13 | 20 |
| Non remission | 4 | 31 | 35 |
| Total | 11 | 44 | 55 |
Risk ratio: 2.15 IC 95% (1.13-4.07)
Table 4: Comparison of the two groups of patients (Bromo versus without Bromo) according to their remission status.
We also found that this improvement also persist over time until the end of 36 months. At 6 months, 27.3% of patients on Bromocriptine had recovered their left ventricular systolic function versus 3.7% on conventional treatment. At 12 months, 63.6% of patients on Bromocriptine had recovered their left ventricular systolic function versus 29.6% on conventional treatment. At 24 months, 75.8% of patients on Bromocriptine had recovered their left ventricular systolic function versus 48.1% on conventional treatment (p=0.036). Bromocriptine, in addition to conventional treatment for heart failure, significantly (p=0.036) favored the recovery of systolic function of the left ventricle. Patients on Bromocriptine would also recover much faster compared to those on conventional heart failure treatment alone, as shown in (Figure 2).
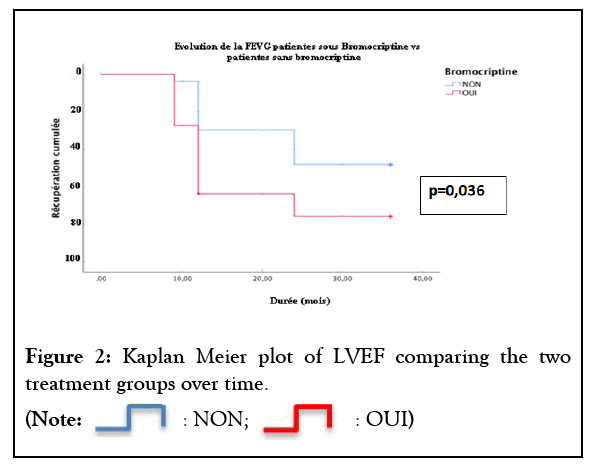
Figure 2 : Kaplan Meier plot of LVEF comparing the two treatment groups over time. 
The aim of this study was to evaluate the long-term efficacy of bromocriptine in the treatment of peripartum cardiomyopathy. This work is a longitudinal prospective cohort study. It compares the efficacy between the reference treatment + bromocriptine vs reference treatment only in patients with peripartum cardiomyopathy with an ejection fraction of less than 35%. An observational cohort of CMPP in Germany found a greater proportion of women exposed to bromocriptine among those who improved their LVEF by 10% or more (59/82; 72%) compared to non-improvers [10].
However, in most studies around the world, the treatment of peripartum cardiomyopathy follows the main principles of the treatment of heart failure with reduced ejection fraction. To date, the adoption of bromocriptine as a legitimate option in the PPCM armamentarium is widely variable across the world, ranging from 67% in Germany to 1% in the United States [11]. In a randomized, princeps study by Sliwa in 2010 [7], the beneficial effect of bromocriptine on LV systolic function and mortality was clearly demonstrated. Indeed, this work noted at the 6th month a significant improvement in LVEF in patients on bromocriptine (LVEF increased from 27 to 58%; p=0.012). However, the sample size was small, only 11 patients.
In Africa where this condition is still common with higher mortality in Europe ranging from 7.4 to 10% [12-15]. Due to the often late discovery and the unavailability of interventional therapies that are too expensive, this molecule is very accessible and affordable and could be a serious option. Yameogo [16] on a population of 48 out of 96 patients, i.e. 50%, found that bromocriptine combined with standard treatment for heart failure leads to rapid and almost complete recovery of left and right ventricular function and reduces mortality associated with this disease. The efficacy of this drug is greater in patients whose symptoms occurred before delivery or in the first month after [7]. In total, these two studies and our had shown the beneficial benefit of Bromocriptine on LV systolic function in PPMCs in subsaharian Africa.
However, some authors have expressed some reserve because the risks of thrombotic complications associated with this treatment, but also because of the consequences for the children of these patients, who are unable to breastfeed [17]. In the absence of certain evidence of its benefit such as large-scale randomized studies, its prescription is still IIb recommendation of the ESC 2019 [1]. In our context, the unavailability of mechanical support treatments for severe forms as well as heart transplantation reflects the importance that this molecule could have in reducing maternal morbidity and mortality in this group of patients with a severe form. Our work shows that Bromocriptine, in addition to the classic treatment of heart failure, would significantly improve (p=0.036) the recovery of systolic function of the left ventricle. Patients on Bromocriptine would also recover much faster compared to those on conventional heart failure treatment alone. Patients on bromocriptine also had a 6-month remission rate twice as high compared to other patients not treated with bromocriptine.
Regarding side effects, one serious adverse event in one patient was reported. This is the pulmonary embolism which occurred after 12 days of treatment, the causality was considered possible with regard to the treatment with bromocriptine. The rate of maternal major adverse events among all patients in the German registry was 10% with a mortality rate of 2%. In the prospective North American IPAC study including PPCM patients with LVEF<45%, a comparable event rate of 7% and a mortality rate of approximately 4% were reported [8].
Our work in addition to finding these results proving the effectiveness of bromocriptine in the short term also shows that the positive effect of bromocriptine is maintained over time since we see that even beyond a follow-up of 3 years the patients under bromocriptine had a better outcome compared to those without bromocriptine. This study is the first work showing the beneficial effect of bromocriptine in the long term. However, due to the small size of our study, these results cannot be considered definitive.
This study presented certain limitations in addition to the small sample size, in particular the number of patients on bromocriptine, which was due to the fact that it was necessary to respect the criteria for placing on bromocriptine or the diagnostic wandering or the long delays in consulting our patients often led to a late diagnosis beyond 1 month. Also in the follow-up, we did not have enough data to make a comparative analysis after 1 month and at 3 months of treatment. Finally, the absence of randomization which means that certain selection biases cannot be excluded.
Peripartum cardiomyopathy is a serious heart condition that worsens maternal mortality in Africa. Bromocriptine improves the short-term and long-term prognosis of these patients. Large cohort clinical trials should be conducted in sub-Saharan Africa to better prove its effectiveness where this condition is more common. It therefore seems desirable to have a definitive answer to this question to implement a multicenter randomized clinical trial involving several countries in sub-Saharan Africa.
Aw Fatou, Sarr SA Leye M, wrote the manuscript, Mingou, JS, Rim, MK, , Kane M, Ndoye G, Diouf MT, Affangla AD, Diouf Y, Diop KR, Beye SM, Ngaïdé AA, Dioum M, Bodian M, followed the patients, Ndiaye MB, Mbaye A, readead the echocardiography and Kane Ad, Kane A, Diao M, Faye A corrected the manuscript. All authors have approved the final article.
[Cross Ref][Google Scholar](All Versions)[Pub Med]
Citation: Aw F, Sarr SA, Mingou JS, Khaled R, Kane M, Gueye N, et al. (2022) Long-Term Effect of Bromocriptine on Left Ventricle Systolic Function Recovery in Peripartum Cardiomyopathy: A Prospective Longitudinal Study. J Clin Exp Cardiolog.13.756.
Received: 25-Nov-2022, Manuscript No. JCEC-22-20433; Editor assigned: 29-Nov-2022, Pre QC No. JCEC-22-20433; Reviewed: 13-Dec-2022, QC No. JCEC-22-20433; Revised: 21-Dec-2022, Manuscript No. JCEC-22-20433; Published: 28-Dec-2022 , DOI: 10.35248/2155-9880.22.13.756
Copyright: © 2022 Aw F, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.