
Anesthesia & Clinical Research
Open Access
ISSN: 2155-6148

ISSN: 2155-6148
Research Article - (2024)Volume 15, Issue 5
Aim: The authors aim to place 1 mg as a standard dose for analgesia after caesarian section.
Background: The anesthesiologist is responsible for an effective analgesia after caesarian section, contributing to the well-being of the mother and the minimization of postoperative complications, such as venous thromboembolism, through implementation of early ambulation. The main purpose of this paper is to point out the evidence of effectiveness in postoperative pain control of elective or urgent caesarian section, with confirmation of lower incidence of adverse effects, using a dose of epidural morphine never tested to date, which is 1/3 of the standard dose used in most centers.
Materials and methods: 50 term parturients undergoing cesarean delivery under epidural anesthesia were enrolled in this study. Patients were randomly allocated to receive either 3 mg or 1 mg epidural morphine. In addition, subjects received regular systemic ketorolac and acetaminophen. Rescue analgesia (iv metamizole) was administered for breakthrough pain. Pain intensity at rest using a verbal response scale (VRS 0-10) was regularly assessed for 48 hours. The primary outcome was pain control at rest (VRS<4/10) 24 hours post-operatively. Secondary outcomes included pain scores at 6, 12 and 48 hours post-operatively (rest/mobilization), incidence of side effects (sedation, nausea/ vomiting, pruritus, urinary retention and ileus) and maternal satisfaction. Statistical analysis was performed with SPSS-statistics for windows (Version 20.0. Armonk, NY: IBM Corp).
Results and discussion: Results showed no significant differences in pain relief at rest within 24 hours. The incidence of nausea, vomiting, pruritus and urinary retention was lower in the 1 mg group and time to recovery of bowel function was shorter. The 1 mg group had higher rates of satisfaction than 3 mg group.
Conclusion: When used as part of a multimodal analgesia regimen, 1 mg epidural morphine provided no inferior post-caesarean section analgesia with fewer adverse effects compared with 3 mg epidural morphine.
Anesthesiologist; Post-caesarean section; Bowel function; Pain relief
Like most other post-surgical populations, the new mother needs effective and adequate pain relief after caesarian section. This subpopulation of patients has even more compelling reasons to achieve optimal postoperative pain relief than other surgical patients, but they are also presented with unique challenges. There is a higher risk for thromboembolic events, which may also be aggravated by immobility from inadequate pain control or excessive sedation from opioids. Moreover, the new mother wants to ambulate, to be alert and energetic enough to care for, interact with and breastfeed their newborn. With these goals in mind and grounding the approach on a multimodal regimen, the analgesic of choice requires minimal transfer in breast milk, little or no effect on neonates, minimal maternal side effects and minimal or no interference with caring for the newborn or discharge from hospital. Improved pain relief may improve postoperative outcomes whereas unsuppressed postoperative pain may lead to amplified pain responses and development of hyperalgesia and chronic pain, of which the incidence after cesarean delivery is ranging from 15% at 3 months to 11% at 12 months [1].
Furthermore, endocrine changes and stress resulting from pain may interfere with lactation. Thus, achievement of adequate pain relief after caesarean delivery is crucial. Neuraxial and systemic opioids are acknowledged standards for post-caesarian delivery pain relief as part as a multimodal approach. Other potentially useful techniques are local wound infiltration or peripheral nerve blockage (e.g. Transverse abdominus plane, quadratus lumborum or ilioinguinal and iliohypogastric nerve block) [2].
Epidurally administered morphine promotes adequate and longlasting postoperative analgesia. However, it may cause side effects such as nausea, vomiting, pruritus, sedation and respiratory depression. The quality of analgesia and incidence of side effects may vary according to the dose of morphine used. The optimal dose capable of providing better analgesia with the lowest incidence of side effects has not yet been defined. A recent trial demonstrated that, when used as part of a multimodal analgesia regimen, 1.5 mg epidural morphine provides noninferior post cesarean section analgesia and causes fewer adverse effects when compared with 3 mg epidural morphine, which was considered the standard dose on our institution.
Therefore, the authors conducted a prospective, randomized, noninferiority trial which the primary outcome was to evaluate the efficacy of the lowest dose of morphine tested to date (1 mg), when compared to standard dose (3 mg) of epidural morphine, for 48 hours after caesarian section. The authors also aimed to evaluate the incidence of side effects related to the administration of opioid and Maternal satisfaction in women proposed to caesarian delivery, with epidural catheter in place, as part as a multimodal analgesia scheme [3].
This prospective, randomized, noninferiority trial took place in the delivery room and postnatal ward of Santa Maria hospital after the approval by the hospital ethics committee. 50 eligible participants of 57 were enrolled for this study with 18-42 years old, randomized by using a list generated electronically to either 3 mg-standard or 1 mg-low, in equitable groups, transferred to individual envelopes. Inclusion criteria comprised: Written informed consent; elective or urgent caesarian section on term parturient; performed under regional anesthesia (combined spinal-epidural or epidural); primiparous or multiparous; first or following pregnancy; age ≥ 18 years; ASA ≤ 3; Pfannenstiel incision; fulfillment of multimodal analgesia scheme. Exclusion criteria were: Post-operative catheter migration before the end of the third administration; difficult anesthetic technique (multiple/hematic/inadvertent dural puncture or paresthesia); absolute or relative contraindication for regional technique; history of chronic pain or consumption of antidepressants or narcotics in the preoperative period; inability to understand the pain scale used; documented intolerance or allergy to any of the drugs used in the postoperative analgesia scheme; obstetric complications in the perioperative period [4].
Group allocation (low vs. standard) was performed in the operating room, after surgery had started, by opening the next sequentially numbered, sealed, opaque envelope containing a computer-generated random code specifying the group assignment. Upon arrival at the operating room, a Ringer’s lactate solution was infused along with Cefoxitin (2 g) for antibiotic prophylaxis and metoclopramide (10 mg) plus ranitidine (50 mg) as aspiration prophylaxis. Standard monitoring included noninvasive arterial blood pressure, pulse oximetry and 3-lead electrocardiogram. If an epidural catheter was already in place for labor analgesia, patients received epidural anesthesia with ropivacaine 0.75% and sufentanil until a T4 sensory level to cold was achieved. Otherwise patients were placed in sitting position and a combined spinal epidural technique was accomplished. Spinal puncture was performed with a 27G pencil point needle, through L3-L4 or L4-L5 intervertebral spaces. For intrathecal anesthesia, 0.75% ropivacaine according to anesthesiologist criteria plus 2.5 mcg of sufentanil were administered. Patients were then positioned supine with left tilt. Intraoperatively the following sequence of drugs was administered intravenously: Acetaminophen 1 g, ketorolac 10 mg, ondansetron 4 mg, droperidol 0.625 mg and oxytocin 5U slow bolus plus 15 U infusion. Vasopressors, IV fluids and sedation complement with propofol were given at the discretion of the attending anesthesiologist [5].
After surgery, the patients were transferred to the Post- Anesthesia Care Unit (PACU) and discharged from after completing 9 points in the modified Aldrete-Kroulik scale. Postoperative analgesic and antiemetic prescriptions were standardized: 3 mg or 1 mg epidural morphine (according to the group) after reversal of motor block followed by 2 administrations of respective dose every 12 hours (both doses were diluted with saline to a total volume of 5 mL), acetaminophen1g IV 3id, ketorolac 10 mg IV 3id, droperidol 0.625 mg IV 3id. As rescue therapeutics, the authors included metamizole 2 g IV, ondansetron 1 mg IV and hydroxyzine 25 mg PO. Follow up occurred at 6, 12, 24 and 48 hours postoperatively, in person, by a member of the LODE team. The primary outcome was pain control at rest evaluated by an 11- point Numerical Rating Scale (NRS) at 24 hours postoperatively (0=no pain; 10=worst pain imaginable) [6].
Secondary outcomes included pain scores at rest at 6, 12 and 48 hours postoperatively, incidence of adverse effects (sedation, nausea/vomiting, pruritus, urinary retention and ileus at 6, 12, 24 and 48 hours PO) and maternal satisfaction.
Pain control was considered successful when evaluation with NRS<4/10 at rest. Sedation was accessed using Ramsay 6-points Sedation scale. Urinary retention was considered when bladder recatheterization was necessary after 24 hours postoperatively and ileus was considered if no bowel movements were present after 48 hours. Maternal satisfaction with pain management was evaluated using 4-point Likert scale.
Calculation of sample size settled 50 patients to have an 80% chance of detecting a decrease in the primary outcome measure from 93% in the control group to 62% in the experimental group (p=0.05). 25 individuals were recruited to each group and a post hoc power analysis to the variables mentioned was performed using the program GraphPad Prism 6.00 (GraphPad Software, Inc. La Jolla, CA). The demographic analysis was performed with student's T test. The primary outcome and maternal satisfaction were analyzed with Mann-Whitney test with a significance level <0.05. The incidence of adverse effects was analyzed with Fisher exact 2-sided test, with similar level of significance [7].
From December of 2014 until April 2015, 57 participants were enrolled for this study. However, 7 were excluded from primary analysis (Figure 1). The groups were homogeneous regarding demographic data (Table 1).

Figure 1: Consort diagram.
| Variables | 3 mg (n=25) | 1 mg (n=25) |
|---|---|---|
| Age (years) | 32 (6) | 34 (7) |
| Current weigh (Kg) | 72 (12) | 78 (11) |
| Height (cm) | 160 (5) | 162 (3) |
| Primiparous/Multiparous | 13/12 (52%/48%) | 9/16 (36%/64%) |
| Previous cesarean delivery | 9 (36%) | 11 (44%) |
Table 1: Demographic data. Values are mean (SD) or n (percentage).
Randomization proved to be suitable for both groups by contingency analysis. The duration of surgery was similar for both groups [8].
Regarding primary outcome, the proportion of patients with successful pain control was 96% for 1 mg group and 80% for 3 mg group. No significant differences on 2-tailed Mann-Whitney U test (P=0.136) were shown in pain relief at rest within 24 hours between low dose and standard dose (Figure 2).
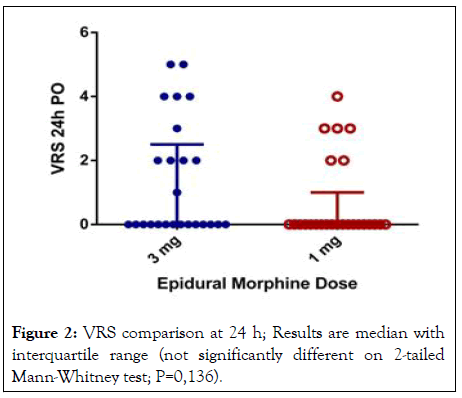
Figure 2: VRS comparison at 24 h; Results are median with interquartile range (not significantly different on 2-tailed Mann-Whitney test; P=0,136).
There were also no statistically significant differences between groups in NRS pain scores at rest during the first 12-hour study period on 2-tailed Mann-Whitney U test (P values for 6 and 12 were 0.20 and 0.19 respectively). Regarding 48 hours evaluation, the results were statistically different due to 1 mg group lower pain scores (P=0.04) (Figure 3) [9].
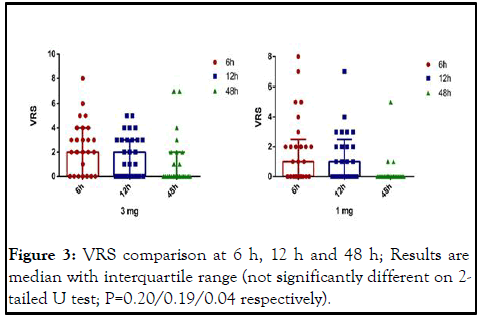
Figure 3: VRS comparison at 6 h, 12 h and 48 h; Results are median with interquartile range (not significantly different on 2- tailed U test; P=0.20/0.19/0.04 respectively).
The incidence of nausea, vomiting, pruritus and urinary retention was lower in the low dose group and time to recovery of bowel function was shorter, being this last analysis statistically significant with a P value of 0.008 (Table 2) [10].
The 1 mg group had higher rates of satisfaction than 3 mg group after 48 hours although this difference was not statistically significant (P=0.34) (Figure 4) [11].
| Variables | 3 mg (n=25) | 1 mg (n=25) | P value |
|---|---|---|---|
| Sedation | 5 | 1 | 0.189 |
| Nausea/Vomiting | 6 | 1 | 0.098 |
| Pruritus | 11 | 5 | 0.128 |
| Urinary retention | 2 | 1 | 1 |
| Gastroparesis 48 h | 11 | 2 | 0.008 |
Table 2: Analysis with fisher exact test.
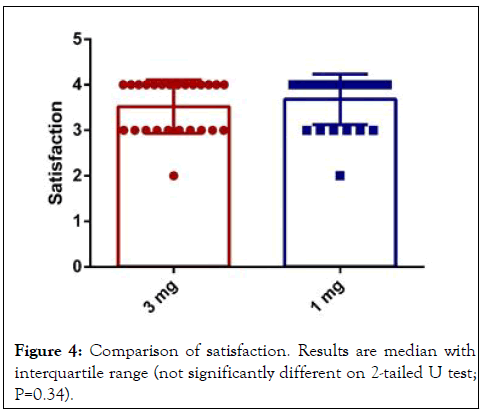
Figure 4: Comparison of satisfaction. Results are median with interquartile range (not significantly different on 2-tailed U test; P=0.34).
Pain assessment owes its complexity to emotional, ethnic, cultural, social and cognitive factors. To standardize and allow this assessment to be more focused, a variety of pain scales were created. The authors elected unidimensional Numerical Rating Scale (NRS) to assess both groups pain scores. This scale takes less than one minute to complete, is easy to administer and score, is transversal across cultures and languages and it can be applied both verbally and in writing [12].
One weakness is that the NRS evaluates only one static component of the pain experience and intensity and therefore does not capture the complexity and idiosyncratic nature of the pain experience or improvements due to symptom fluctuations. The authors registered also the requirement of rescue analgesia as an alternative to measure the effectiveness of analgesia. Opioid based analgesia, as part of a multimodal regimen, is considered a gold standard in clinical practice for the treatment of postoperative pain and morphine is one of the most common used opioids in the perioperative setting, especially in obstetrics. Opioids are often added to neuraxial local anesthetics in patients undergoing surgery without general anesthesia and in some institutions an opioid alone, typically morphine, is administered intrathecally as a single-dose injection in labor analgesia. On the authors institution intrathecal morphine is not routinely used. Instead the post-operative analgesia prescription includes one bolus of epidural morphine every 12 hours, typically (before this study) 3 mg, besides multimodal regimen [13].
Morphine is relatively lipid insoluble, which accounts for its slow penetration of the dura and hence its slow onset 45-60 minutes. Its duration of action is long (8 to 24 hours) because it persists in the liquor. Morphine appears in the liquor within 15 minutes and reaches peak concentration at 90-120 minutes. Analgesia results from spinal uptake rather than systemic effects because lower doses offer superior analgesia than intramuscular, intravenous and patient controlled analgesia techniques which attain higher plasma levels of morphine. Side effects of neuraxial morphine even at reduced doses include nausea and vomiting, sedation, respiratory depression, as with parenteral routes, but to these can be added pruritus (10%-50%) and urinary retention (5%-40%), depending on which studies are reviewed. Pruritus is not due to histamine release but may respond to antihistamines and it will always respond to a small dose of naloxone without reducing analgesia.
Concerning the primary outcome in this study, the authors found that the use of 1 mg and 3 mg of epidural morphine did not show statistically significant difference in pain scores at 24 h evaluation between both groups of patients who underwent caesarean section. This result showed that there is no direct relationship between the dose of neuraxial morphine and quality of analgesia, which are consistent with the results of other authors. Pain intensity in the first hours after anesthesia is less due to the residual effect of local anesthetic, which certainly reduced the mean pain for 24 hours. Regarding the secondary outcomes of this study, in the present trial the authors decided to evaluate pain scores on three other occasions: 6, 12 and 48 h post-operative, concluding the quality and duration of analgesia was superior with 1 mg of epidural morphine. The main hypothesis for this result accounts for the fewer incidence of side effects in the 1mg group vs. 3 mg group, which might contribute for multidimensional perception of pain and mother satisfaction.
The incidence of pruritus, sedation, nausea/vomiting and urinary retention was not statistically different between groups, however the absolute number was superior in the 3 mg group and the authors hypothesize that this number could not reach statistical significance due to a small population study. Pruritus was the most common side effect after the use of epidural morphine in both groups and had variable incidence, which in the obstetric population may be more frequent due to the interaction with estrogen receptors Mu (μ). Although there were some cases of sedation according to the Ramsay Sedation Score, no case of respiratory depression was noticed, which does not mean that bradypnea did not occurred. Despite the reported sedation cases in the parturient, no respiratory intervention was needed (supplementation of oxygen, naloxone administration or others).
Morphine administered systemically and also epidurally, is known to delay gastrointestinal transit. In this trial, the incidence of bowel dysfunction was statistically superior in the 3 mg group (p=0.008). The clinical consequences of postoperative bowel dysfunction after caesarean section are intestinal gas retention, abdominal distension, nausea and abdominal pain, which can play a role in the higher pain scores observed in the 3 mg group at 48 h.
When used as part of a multimodal analgesia regimen, 1 mg epidural morphine compared with 3 mg epidural morphine, offered the advantage of superior quality and duration of analgesia in post-caesarean section analgesia with fewer side effects. There are some limitations in this study. First, data on primary technique for labor analgesia (combined spinal-epidural or epidural alone) was not recorded which can modify the perception of subsequent pain. Also, clinician-administered boluses during labor, duration of labor, final pain score prior to cesarean delivery, degree of urgency, uterine externalization, anesthesiologists experience, resident or attending physician responsible for anesthetic induction, testing method for block quality prior to surgery, as well as time of and reason for conversion to GA or the second anesthesia were not collected and analyzed in this study. Another limitation was the dose and analgesia scheme selection. The authors selected the most common dose used and decided to compare it with a reasonable dose for efficacy, rather than a variety of doses, thus is not possible to make conclusions about other dose regimens. More studies should be carried to validate this conclusions.
None.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Araujo R, et al. (2024) Low Dose Epidural Morphine after Caesarian Section as Effective as Standard Dose: A Randomized Controlled Trial. J Anesth Clin Res. 15:1150.
Received: 17-Apr-2020, Manuscript No. JACR-24-3905; Editor assigned: 22-Apr-2020, Pre QC No. JACR-24-3905 (PQ); Reviewed: 06-May-2020, QC No. JACR-24-3905; Revised: 03-Jun-2024, Manuscript No. JACR-24-3905 (R); Published: 28-Oct-2024 , DOI: 10.35248/2155-6148.24.15.1161
Copyright: © 2024 Araujo R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.