Journal of Osteoporosis and Physical Activity
Open Access
ISSN: 2329-9509
ISSN: 2329-9509
Case Report - (2022)Volume 10, Issue 2
Objective: We reported recently on the effect of the LION procedure for recovery some walking functions in SCI peoples. The objectives of the present studies were to determine the effect of the low-frequency stimulation of the pelvic nerves on autonomic functions.
Design: Observational case report.
Setting: Tertiary referral unit specialized in neuropelveology.
Participants: Five patients with chronic Spinal Cord Injury (SCI) who underwent a LION procedure to the pelvic somatic nerves for the recovery of standing and walking motion. Intervention: Patients underwent continuous low-frequency pelvic lumbosacral nerve neuromodulation. Main Outcome Measures: DXA scans, plethysmography and lean body mass of the lower limbs before and at follow-up two years after the LION procedure.
Results: The lower limb lean body mass increased in all five patients from 17.5 in average (± 4.45; Min 12.6-Max 23.3) directly after implantation to 23.22 (± 6.46; Min 16.1-Max 31.92), P<0.01 at 24-months follow-up, corresponding to an increase of 32% in average (± 0.033; Min 28%-Max 37%). The plethysmography examination showed in all 5 patients without any exception an instantaneous and explosive increase of Flow (ml/s) from a based line around 100 UI to >400 UI starting as soon the stimulation was turned on. Finally, the evolution of the T-score before starting with stimulation was in average -2.4 (± 0.96; Max-3, Min-1) and increased to -1.0 (± 0.57; Max-1.5, Min 0), P<0.01 at 2-years follow-up, suggesting significant improvement of bone mineral density after pelvic nerves stimulation.
Conclusions: The stimulation of pelvic sympathetic fibers should theoretically induce a peripheral vascular contraction with a reduction of the bone density of the lower limbs. However, the present study shows completely opposite results with a much higher muscle mass building, a peripheral vasodilatation as well as a significant improvement of the bones mineral density.
Spinal cord injury; LION procedure; Sympathetic nervous system; Functional electrical stimulation; Osteoporosis; Rehabilitation
BMD: Bone Mineral density; FES: Functional Electrical Stimulation; SCI: Spinal Cord Injury; LION: Laparoscopic Implantation of Neuroprosthesis; vs.: Versus; DEXA: Dual-Energy X-ray Absorptiometry; LBM: Lean Body Mass; LL-LBM: Lower Limb Lean Body Mass
Osteoporosis and muscle atrophy are frequently cited complications occurring after a Spinal Cord Injury (SCI) [1,2] and long-bed rest [3,4]. After SCI, there is a rapid and dramatic loss of muscle mass below the level of the lesion [5] with up to 14%-16% decreases in quadriceps, hamstrings and adductor muscles from 6 to 24 weeks post injury [6]. Increased spasticity may help to preserve muscle mass in individuals with SCI, not bones [5]. After SCI, notable increases in bone desorption markers have been reported to occur as early as 2 weeks, reaching peak values 2 to 4 months after injury onset [7,8]. In general, hip BMD declines rapidly for the first several months, and then declines more slowly until reaching equilibrium at 12 to 16 months post injury. Lumbar spine BMD generally does not decline and may even increase after SCI [9]. Therefore lowenergy fractures in individuals with SCI are classical complications that may occur during events that would not normally cause fracture (fracture rate in the SCI population 1%-21%) [10]. Complications related to fracture in the SCI population such as altered fracture healing, delayed union, mal or nonunion, pressure sores, infection and osteomyelitis, present an additional source of morbidity [11]. In turn, hypercalciuria is often reported after SCI; ionized calcium has been demonstrated to increase into the hypercalcemic range after SCI with a parallel increase in urinary calcium excretion representing a potential risk of kidney stones formation [12].
In a recently published study on the LION procedure in chronic paralyzers [13], we reported that the stimulation of the pelvic somatic nerves might obviously induce the building of muscle mass, which constitutes a major step towards preventing the formation of decubitus lesions [14]. We also mentioned based on our clinical observations that treatment may result in an increase in cutaneous temperature in the stimulated areas, especially in the gluteal region [15]. We postulated that the observed “side effects” might be due to pelvic nerve lowfrequency stimulation [16], which increases transcutaneous oxygen pressure and enhances new blood vessel formation (angiogenesis) [17]. When combined with locomotors training (gluteal contractions) and the stimulation-induced building of gluteal muscle mass (“gluteal pads effect”), as described above, this method provides better protection against decubitus ulcers, deep venous thrombosis, and edema [18]. One of the major fears we had at the beginning of the study was the risk of bone fracture during walking training due to SCI-inducedosteoporosis. Our fear was not confirmed insofar as none of our patients presented any bone fracture in spite of intensive walking up to 2.6 km in our best patient.
In this context, the purpose of this manuscript is to bring the attention of the scientific community on a possible revolutionary effect of pelvic nerves stimulation LION procedure for preventing or reversing osteoporosis and muscle mass loss in spinal cord injured peoples, which findings may found new applications for treatment these conditions in global human population on earth and in microgravity.
Participants
This manuscript is a part in continuation of the previous publication of 10-years’ experience with LION procedure in SCI peoples [13]. We have examined the value of these observations in the same 26 consecutive patients with SCI levels varying between C6 and Th12 (tetraplegics n=3, paraplegics n=23) with ASI Grade A, n=13, Grade B, n=11 and AIS Grade C, n=2.
All patients who took part in this study signed informed consent forms prior to surgery and provided written informed consent for the use and publication of case details, personal information’s, execution and evaluation of the present investigations, images and videos. This study was done in accordance with the 1975 Declaration of Helsinki and with ethics approval from Medical Ethics Committee of our Institution.
Evaluation of muscle mass
To analyze the changes in muscles masse, we measured in regularly intervals with a tape measure the circumference of both tight at the midpoint between the anterior superior iliac spine and the knee joint line in all recruited patients. In addition, in five subjects the lower limb Lean Body Mass (LBM) was measured at begin with the stimulation and after 1 year of training by a Lunar DPX dual energy X-ray absorptiometry (Madison, WI).
Assessment of blood circulation in the lower extremities
To assess to blood circulation in the lower extremities, we used three different methods (without vs. with stimulation at 10 Hz/90 μs/1 mA of the somatic pelvic nerves-sciatic and femoral nerves):
The skin temperature was measured by a digital infrared thermometer, non-contact laser IR temperature Gun instant at the anterior face of the thigh first without stimulation, and 5 minutes after starting stimulation in all 29 patients.
Photography’s of the lower limbs by a FLIR T335 infrared hot camera with and without stimulation in 5 patients.
To analyze either the temperature increase is related to the underlying muscle activity or to an increased blood flow; we performed a lower limb laser plethysmography with infrared camera in 5 patients.
Measurement of bone density
For diagnostic evaluation level of osteoporosis, in 5 patients we used a classical Dual-Energy X-ray Absorptiometry (DEXA) for the hip between the femoral neck and total hip (T scores: normal ≥ -1DS, -1DS <...> -2.5DS osteopenia and -≤-2.5DS osteoporosis) before the LION procedure and at 2-years followup.
Statistical analyses were performed using a paired Student’s ttest. Differences were considered significant for p ≤ 0.05 (*), and p ≤ 0.01 (**) if not noted otherwise.
Muscle mass
Immediately after implantation, stimulation at a frequency lower than 40 Hz induces in all patients muscle fasciculation’s with a saccadic extension of the knee followed by a slow release of this extension starting after a few seconds in spite of the persistence of the stimulation at the same intensity of current. After six months of training, this same stimulation frequency could be reduced to 20-30 Hz with a harmonious extension of the leg, which can last 1 minute or more. Thigh diameter measurements increased in all 26 patients by an average of 3 cm (± 2, 1-4 cm).
The lower limb lean body mass was performed in five paraplegic patients (AIS Grade A n=4, AIS Grade B n=1). The Lower Limb Lean Body Mass (LL-LBM) increased in all five patients from 17.5 in average (± 4.45; Min 12.6-Max 23.3) directly after implantation to 23.22 (± 6.46; Min 16.1 – Max 31.92), P<0.01 at 24-months follow-up, corresponding to an increase of 32% in average (± 0.033; Min 28%-Max 37%). Thus, the increase of thigh diameter stems not from fat deposition, but rather from muscle mass gain. The change was significant for all five patients (Figure 1).
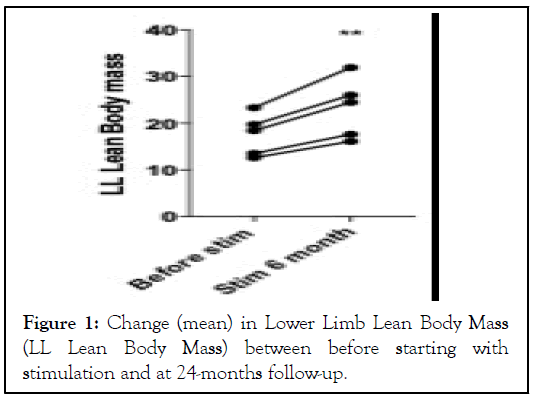
Figure 1: Change (mean) in Lower Limb Lean Body Mass (LL Lean Body Mass) between before starting with stimulation and at 24-months follow-up.
Blood circulation in the lower extremities
Skin temperature: Room temperature averaged 24.2 ± 0.9°. After 5 minutes of stimulation, an increase of skin temperature of 0.8°C (± 0.3) in average was noted in all 29 patients. The increase in temperatures in the lower extremities has been further confirmed with the infrared hot camera in five patients (Figure 2).
Figure 2: Infrared hot camera photo of the lower limbs – left: stimulation “off” – right: stimulation “on”.
The plethysmography examination showed in all 5 patients without any exception an instantaneous and explosive increase of flow (ml/s) from a based line around 100 UI to >400 UI starting as soon the stimulation was turned on. Also the color of the LL changes immediately from blue color to dark red instantaneously in all 5 patients (Figures 3a and 3b).
Figure 3: Plethysmography of the lower limbs – left (a): stimulation “off” – right (b): stimulation “on”.
Together these data indicate that low-frequency stimulation of pelvic nerves enhances the low extremities temperature by increasing the blood flow.
Osteoporosis
No patient in this study or in our previous studies had a bone fracture of the lower limbs or spine. The evolution of the T-score before starting with stimulation was in average -2.4 (± 0.96; Max -3, Min -1) and increased to -1.0 (± 0.57; Max -1.5, Min 0), P<0.01 at 2-years follow-up (Fig. 4), suggesting significant improvement of bone mineral density after pelvic nerves stimulation.
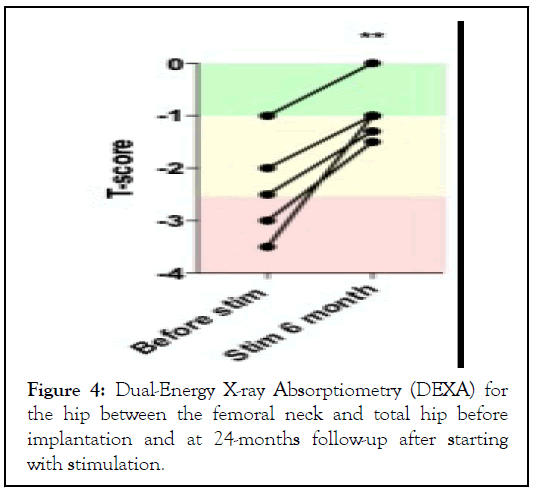
Figure 4: Dual-Energy X-ray Absorptiometry (DEXA) for the hip between the femoral neck and total hip before implantation and at 24-months follow-up after starting with stimulation.
There is considerable debate as to the clinical benefits of Functional Electrical Stimulation (FES) in the treatment of SCI. Previous studies conducted in chronically paralyzed individuals have shown only moderate improvement in muscle mass. Baldi, et al. showed that FES-cycle ergometer (FES-CE) could prevent disuse atrophy when training is initiated during the acute phase of muscle atrophy resulting from SCI [19]. In the same report they mentioned an increase of LL-LBM by 9.3% at 6-months follow-up. Both FES and the LION procedure enable an “electrically-induced” muscle contraction. While FES generates an “on-off” muscle contraction, nerves stimulation generates a harmonious, modular and more physiological muscles contraction. In LION-program of rehabilitation 15, in addition to the phases of “electrically-assisted” training, pelvic somatic nerves are continuously stimulated with a low-frequency electrical current at the lowest intensity level needed for the first minimal visible skeletal muscle contraction. This “underlying low-frequency stimulation” enables a permanent stimulation of the muscles at such a level where no muscle contractions are observed, but is not less effective in term of muscle building. This capability of non-stop stimulation via an “in-body trainer” may obviously explain the exceptional results reported here with an increase in LL-LBM of 32% in average. Such an increased lower limb/gluteal muscle mass, combined with standing and walking exercises may decrease seating pressures and reduce in consequence the prevalence of pressure scores.
The annual incidence of pressure ulcer formation in spinal cord injured peoples varies between 23% and 30% according to the literature, and more than 85% of patients will develop at least one pressure ulcer in their lifetime. Pressure ulcers constitute nearly 20% of the reasons for re-hospitalization in rehabilitation centers. Building of gluteal muscle mass in combination with increased peripheral vasodilation resulting in a significant increase in transcutaneous oxygen pressures and lifting/walking exercises due to stimulation provides a triple benefit for pressure ulcer prevention.
The continuous low-frequency/low-intensity stimulation of the pelvic somatic nerves may enable a passive and not perceptible efficient muscles training not only in paraplegics but also in every individual. This “in-body-trainer” may open the door to a whole new area of humanity in which implanted electronics may help the human body to a better performance. The process of ageing, also called sarcopenia [20], is characterized by muscle atrophy along with a reduction in muscle tissue quality characterized by such factors as the replacement of muscle fibers with fat and the degeneration of the neuromuscular junction leading to a progressive loss of muscle function and frailty. Sarcopenia is responsible for considerable healthcare expenditure, with direct medical costs attributable to the disorder estimated at US $18.5 billion in the United Stated in 2000 [21]. Rates of sarcopenia of between 1% and 29% have been reported in community-dwelling populations and of 14%-33% in residents requiring long-term care [22]. Nutrition (protein intake) has been identified as having an important influence on the development of sarcopenia, but does not appear to be as influential as in maintaining muscle strength or physical function. Physical activity, in particular resistance training, when performed at higher intensities appears beneficial for muscle strength and functioning. The option of an “in-body-trainer” with continuous passive stimulation of the pelvic somatic nerves may become an option in the future for slowing down the aging process by preserving body muscle mass. This technique may be appropriate in elderly people who are not capable of active muscle training because of pain, motoric limitations or subcortical pathologies but also in people confined to bed for long periods of time (prophylaxis of decubitus) [23].
If observed effects of electrical stimulation on muscle mass were expected, effects observed on blood circulation and bone density were more than surprising. Utilization of mechanical stress (forces-standing) induces bone formation, but not in such amount to explain our findings. Improved capillary supply to skeletal muscle after electrical stimulation has been reported [24-26] but effects of stimulation on bone density are rare in the literature. This study presents the first results on the efficacy of the LION procedure in terms of muscle growth in the legs and regain of legs bone mineralization. Our study has limitations, that of not being randomized, as well as the small number of patients (no preoperative references values in most patients).
Sympathetic innervation of the lower limbs originates in the lumbar plexus that supplies the femoral and deep saphenous nerves to the femur, and the tibial, medial, and popliteal nerves to the tibia and fibula. The pelvic parasympathetic nerves mainly supply cholinergic terminals to structures involved in excretory and reproductive functions; however, they have been detected within the bone microenvironment, via immunoreactivity against VAChT and ChAT [27,28]. The main particularity of the LION procedure is that placement of stimulation’s electrodes to the sciatic and femoral nerves enable mainly stimulation of sympathetic fibers anastomosing from the sacral sympathetic trunks. Scientific findings of the sympathetic innervation of bone tissue and its role in the regulation of bone modeling, is of major interest in situations where uncoupling between osteoclasts and osteoblasts occurs [29,30]. By stimulation of these sympathetic fibers, we would rather supposed to observe a peripheral vasoconstriction of resistance arteries, increasing cardiac activity, a reduction of venous capacity and a decrease in bone mineral density as a result of activation of alpha-1 adrenergic receptors by norepinephrine released by post-ganglionic sympathetic neurons [31]. However, the observation reported here seems to show exactly the opposite. It is impossible to determine from the present observations whether this effect related to the simple stimulation of the pelvic nerves, or whether it is related to the low-frequency stimulation of these nerves. The second alternative appears the more plausible: Shyu, et al. have demonstrated that in rats under chloralose anesthesia combined with muscle paralysis with Flaxedil, low-frequency stimulation at 3 Hz of the sciatic nerve induced a long-lasting post-stimulatory vasodilation with decrease in blood pressure due to central inhibition of sympathetic activity [32]. Muzquiz, et al. demonstrated also recently that a reversible low frequency alternating current produced a high degree of nerve block at currents levels comparable to pulse stimulation amplitudes to activate nerves [33].
On the other hand, stimulation of the sciatic nerves induces stimulation of parasympathetic nerve fibers originating respectively from the sacral nerves as well. There are multiple observations to support a possible action of cholinergic signaling (parasympathetic) on bone remodeling as well. Those include the expression of cholinergic signaling molecules in bone cells, the presence of immunoreactivity for cholinergic nerve fibers within the bone microenvironment, the likely interplay between sympathetic and parasympathetic nervous systems for their action on bone remodeling, as well as preclinical and clinical observations [34].
Investigations in progress may provide the answer. The question is of certain interest, since this could have important consequences in prevention or even treatment of osteoporosis on hearth, which condition impacts worldwide by aging causes more than 8.9 million fractures annually (one fracture every 3 s) [35] and affecting 200 million women worldwide (Cost in the US: $13.8 billion/year).
The question is even of major importance for the Mars mission, since this could have important consequences in devising countermeasures to spaceflight induced bone loss. Osteoporosis occurring as a result of microgravity is, from the perspective of the organism down to the lowest biological level, different than that encountered on earth. Microgravity appears to significantly alter the cellular cytoskeleton [36] responsible systematically for osteoporosis coupled with an environment of nearly nonexistent mechanical stress (alteration of the Wolff’s Law). Comparing to osteoporosis on earth, in microgravity, the loss of calcified bony tissue occurs at four times the rate (around 2% per month), does not appear to level off, and appears to be much less reversible. Thus, a three-year trip to Mars is estimated to potentially results in a devastating greater than 50% reduction of bone mass [37]. To date, countermeasures mainly consist in exercise and supplementation with bisphosphonate, but are not enough to maintain bone homeostasis. The higher calcium levels are probably contributing to the increased calcium-stone forming potential. Stones can usually be passed, painfully, without surgery. Drinking plenty of water both helps pass the stones, and prevents them forming. Urinating in toilets in orbit is time consuming, crewmembers are very busy, and if they do extravehicular activity they don't have the option. Vomiting because of motion sickness can also lead to loss of fluids. Kidney stones could prove to be the "final frontier" astronauts embarking on long distance missions have to tackle. Much work is needed to find ways of preventing space travelers developing the stones on long trips.
Decreases in muscle activity and mechanical loading results in muscle atrophy, as has been also observed following spaceflight. Since the earliest days of human spaceflight, physiologists and NASA flight surgeons recognized the importance of exercise to maintain musculoskeletal with exercise up to four hours a day; the overhead of spending several hours every day in space for the sole purpose of exercise is problematic. The mentioned option of an “in-body-trainer” would enable to reduce considerably periods of exercises and may reduce at same time the process of osteoporosis, similarly as in paraplegics, and would represents in turn a potential prophylactic for kidney stone formation in microgravity.
The stimulation of pelvic sympathetic fibers should theoretically induce a peripheral vascular contraction with a reduction of the bone density of the lower limbs. However, the present study shows completely opposite results with a much higher muscle mass building than with traditional rehabilitation methods, a peripheral vasodilatation as well as a significant improvement of the bones mineral density. The discovery of these effects of lowfrequency stimulation on the pelvic vegetative nervous system could have revolutionary therapeutic impact both in the treatment of sarcopenia and in the treatment of osteoporosis on earth and in space.
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
Citation: Possover M (2022) Low Frequency Pelvic Nerves Stimulation: Cutaneous Vasodilation and Retoration of Bone Density in Chronic Spinal Cord Injured People. J Osteopor Phys Act. 10: 293.
Received: 16-Mar-2022, Manuscript No. JOPA-22-16280; Editor assigned: 18-Mar-2022, Pre QC No. JOPA-22-16280 (PQ); Reviewed: 01-Apr-2022, QC No. JOPA-22-16280; Revised: 06-Apr-2022, Manuscript No. JOPA-22-16280 (R); Published: 13-Apr-2022 , DOI: 10.35841/2329-9509.22.10.293
Copyright: © 2022 Possover M. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.