Enzyme Engineering
Open Access
ISSN: 2329-6674
ISSN: 2329-6674
Research Article - (2015) Volume 4, Issue 2
CALB: Candida Antarctica Lipase B; CLD: Crosslink Density; TBU: Tributyrin Hydrolysis Activity Unit
Lipases (triacylglycerol ester hydrolases E.C. 3.1.1.3) stand amongst the most widely used enzymes in biocatalysis, carrying out a wide range of enantio- and regioselective reactions of hydrolysis, esterification, inter-esterification, alcoholysis, acidolysis and aminolysis. Lipases find use in a variety of biotechnological fields such as food and dairy (cheese ripening, flavour development), detergent (lipid-stain digesters), pharmaceutical (naproxen, ibuprofen), agrochemical (insecticide, pesticide), fine chemical and oleochemical (fat and oil hydrolysis, biosurfactants) industries [1-3].
Candida antarctica lipase B (CALB) is an interesting lipase with potential application in a number of industrial processes such as synthesis of triglycerides [4], esterification of terpenic alcohols [5], regioselective esterification of sugars [6], nucleosides [7], steroids [8] and enantioselective resolution of secondary alcohols via hydrolysis [9] or esterification in organic solvents [10]. Chau et al. studied synthesis of sialic acid through the lipase-catalyzed esterification [11]. Habeych et al. reported the synthesis polyesters of linear ester oligomers (LEOs) and cyclic ester oligomers (CEOs) [12]. Yadav et al. reported the transesterificaiton of butyl-4-methyl-3-oxopentanoate with n-butanol [13,14].
Immobilization of CALB on various support carriers have been reported including octyl sepabeads, polyethyleneimine (PEI) agarose, glyoxyl-agarose, glutaraldehyde-agarose and Eupergit-Cu [15], epoxy activated macroporous poly(methyl methacrylate) beads and nanobeads with poly(glycidyl methacrylate) as outer region [16], aminated silica gel and cross-linking the adsorbed enzyme with glutaraldehyde in the presence of detergent [17], epoxy functional beads of poly(glycidyl methacrylate-co-ethylene glycol dimethacrylate) [18], porous styrene-divinyl benzene beads [19].
Commercially available immobilized preparation of CALB namely, Novozym 435 is based on adsorption of CALB onto Lewatit VP OC 1600, a copolymer of poly(methyl methacrylate-co-divinylbenzene) and has average values of particle size, surface area and pore diameter of 315-1000 μ, 130 m2/g and 150 Ǻ respectively. However, there are reports on the physical leaching of CALB from Novozym 435 during reactions (unpublished results) [16]. This necessitates the need to develop new, stable alternative CALB immobilized systems which are comparable to commercially available enzymes like Novozym 435.
Hydrophobic supports can be used to obtain the open and stabilized form of the immobilized lipases even at low ionic strength [20,21]. One of the interesting hydrophobic polymer systems for enzyme immobilization is poly(vinyl acetate-co-divinyl benzene) [poly(VAcco- DVB)]copolymer beads which was studied in the literature.
Jianguo et al. [22], reported the use of poly(VAc-co-DVB) copolymer beads after saponification and activation with β-sulfatoethylsulfonyl aniline (SESA), glutaraldehyde and p-benzoquinone for immobilization of Penicillin G acylase. Guo et al. [23], immobilized Candida cylindracea lipase onto magnetic poly(VAc-co-DVB) beads synthesized by copolymerization of vinyl acetate and divinyl benzene after encapsulating nanomer-sized magnetite (Fe3O4).
In the above two approaches, there is a need to suitably modify the poly(VAc-co-DVB) beads in order to bind the enzyme, like saponification and suitable cross-linking, which makes the process cumbersome and hence not attractive from commercial perspective. The aim of this research paper is to evaluate the possibility of using the poly(VAc-co-DVB) copolymer beads directly for enzyme immobilization without any modification and comparing it with modifications like saponification as well as with Lewatit VP OC 1600. In addition, the beads were characterized by scanning electron microscopy, mercury porosimetry and particle size analyzer to have additional insights of the enzyme binding mechanism. Of the six CALB immobilized copolymer beads with different CLDs, one variation with maximum tributyrin hydrolysis activity (PVAc-DVB-50-UH) was taken up for further study on storage stability as a model system. and compared with the CALB immobilized on Lewatit VP OC 1600.
Materials
Generic CALB enzyme, namely Fermase CALB L, prepared by recombinant Pichia pastoris fermentation was a kind gift from Fermenta Biotech Ltd, India. Fermase CALB L has tributyrin hydrolysis activity units (TBU) of 1350 TBU/mL and a specific activity of 265.75 TBU /mg protein. Poly(vinyl pyrrolidone) (PVP) was procured from BASF, Germany. Tributyrin (97%) was purchased from Sigma-Aldrich. Bovine serum albumin (BSA) was procured from Himedia, Mumbai. All other chemicals such as vinyl acetate (VAc), divinyl benzene, 65% (w/v) (DVB), cyclohexanol, benzoyl peroxide (BPO), iso-propyl alcohol (IPA), sodium hydroxide, buffer salts and methanol were of analytical grade, purchased from Merck, India unless otherwise specified and used as supplied.
Synthesis of copolymer beads
Porous poly(VAc-co-DVB) copolymer beads were synthesized by suspension polymerization in a jacketed cylindrical polymerization glass reactor [24]. The continuous phase comprised of 210 mL of 1.7% (w/v) aqueous solution of PVP. The discontinuous organic phase consisted of total 60 g of two monomers, namely VAc and DVB, where in DVB was a cross-linking monomer. Cyclohexanol was used as a porogen in 1:1 (w/w) ratio with respect to the total monomer weight. 5 g of BPO was used as initiator. A series of copolymer beads of different cross-link densities (CLD) were synthesized at a constant volume of porogen. The CLD of copolymer beads is defined as a percentile molar ratio of cross-linking agent to monomer.
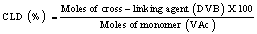
The composition of poly(VAc-co-DVB) copolymer beads is summarized in Table 1.
| Polymer name | Vinyl acetate | Divinyl benzene | % CLD | ||
|---|---|---|---|---|---|
| Weight, g | Moles | Weight, g | Moles | ||
| PVAc-DVB-25-UH | 21.75 | 0.252 | 8.25 | 0.063 | 25 |
| PVAc-DVB-50-UH | 17.03 | 0.197 | 12.97 | 0.099 | 50 |
| PVAc-DVB-75-UH | 14.0 | 0.162 | 16.0 | 0.122 | 75 |
| PVAc-DVB-100-UH | 11.89 | 0.138 | 18.11 | 0.139 | 100 |
| PVAc-DVB-150-UH | 9.136 | 0.106 | 20.87 | 0.160 | 150 |
| PVAc-DVB-200-UH | 7.4 | 0.085 | 22.6 | 0.173 | 200 |
Table 1: Composition of poly(VAc-co-DVB) copolymer beads.
Polymerization reaction was conducted isothermally at 70 oC under a nitrogen overlay by circulating water through the jacket for 5 h at 200 rpm. The copolymer beads obtained were filtered, thoroughly washed with water and soaked in methanol overnight. The beads were air dried and then sieved through 300-500 μm sieves before using for saponification and enzyme immobilization. These copolymer beads without saponification were termed as PVAc-DVB-25-UH, PVAc- DVB-50-UH, PVAc-DVB-75-UH, PVAc-DVB-100-UH, PVAc-DVB- 150-UH, and PVAc-DVB-200-UH depending on their CLD.
Saponification of copolymer beads
5 g of dry and sieved poly(VAc-co-DVB) copolymer beads with different CLD were subjected to partial saponification with methanol containing 4.4% (w/v) sodium hydroxide at 28°C at 150 rpm for 8 h. Saponified polymer beads were washed thoroughly with plenty of water till the pH of elutriate becomes neutral and then air-dried prior to enzyme immobilization. These copolymer beads with saponification were termed as PVAc-DVB-25-8H, PVAc-DVB-50-8H, PVAc-DVB- 75-8H, PVAc-DVB-100-8H, PVAc-DVB-150-8H, PVAc-DVB-200- 8H, wherein 8H denotes the time of hydrolysis as 8 h.
Enzyme immobilization
CALB was immobilized onto poly(VAc-co-DVB) copolymer beads, their saponified counterparts and Lewatit VP OC 1600. Immobilization was done with enzyme activity loading of 10,000 TBU/g of dry polymer beads, based on our earlier work (unpublished data). 5 g of dry polymer beads were incubated with immobilization solution containing 37 mL of Fermase CALB L enzyme (equivalent of 10,000 TBU activity), 25% (v/v) of IPA and 12.5% (v/v) of glycerol at 25°C for 24 h at 150 rpm in an orbital shaker. After incubation, CALB immobilized copolymer beads were separated by vacuum filtration, washed with IPA (25 mL thrice) and air dried. CALB immobilized copolymer beads were stored at 5°C until further use. All immobilization experiments were done in duplicates under similar conditions.
Storage stability of CALB immobilized copolymer beads
CALB immobilized copolymer beads with maximum activity (PVAc-DVB-50-UH) and CALB immobilized Lewatit VP OC 1600 copolymer beads were stored in air-tight containers at 5°C. Storage stability was evaluated by determining the enzyme activity of CALB immobilized copolymer beads at different time intervals up to 100 days.
Analytical methods
As a general rule, most experiments were carried out in triplicate and average values are represented in results. The correlation analysis was done by using Karl-Pearson coefficient.
Copolymer characterization: The average particle diameter of poly(VAc-co-DVB) copolymer beads was determined by laser diffraction analyzer, HELOS H1004 (Germany). Porous properties of beads were determined by mercury intrusion porosimetry using Fisons Instruments Pascal 140/240 porosimeter in the range of 0-4000 kg.cm-2. Shape and surface morphology of poly(VAc-co-DVB) copolymer beads was observed using SEM. Specimen was prepared by mounting dried copolymer beads on stubs and then sputter-coated with gold. Micrographs were taken on a JEOL JSM-5200 (Tokyo) SEM instrument.
Enzyme activity assay: Activity of free and immobilized CALB samples was determined by tributyrin hydrolysis assay titrimetrically using pH STAT (Spectralab AT 38C, India) at pH 7.5 and 40°C using 10% (v/v) tributyrin as a substrate and expressed in Tributyrin hydrolysis units (TBU). One TBU unit of enzyme activity was defined as the amount of enzyme catalyzing the formation of 1 μmmol of free fatty acid from 10% (v/v) tributyrin in the milieu of 100 mM sodium phosphate buffer at pH 7.5 at 40°C. Enzyme activity of CALB immobilized copolymer beads was calculated by using the below mentioned formula. Since enzyme activity measurements vary with respect to the water content, activity is always converted and expressed on dry weight basis.
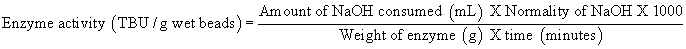
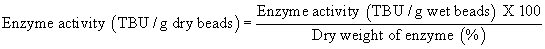
Immobilization Yield was evaluated in terms of protein binding [%] and Activity expression [%] by using the below mentioned formula.
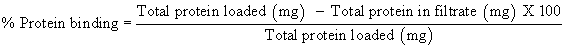
% Activity expression indicates the percentile ratio of active and accessible enzyme with respect to the total enzyme bound on the polymer beads. As in the case of porous polymer matrix, enzyme molecules get bound to polymers either by simple adsorption on surface or by covalent binding deep inside the pores.
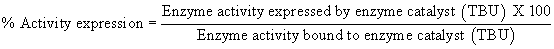
Wherein,
Enzyme activity expressed by enzyme catalyst [TBU]=Enzyme activity of enzyme catalyst (TBU/g wet beads) x weight of enzyme catalyst (g).
Enzyme activity bound to enzyme catalyst [TBU]=Total enzyme activity loaded (TBU) – Total enzyme activity in the filtrate (TBU).
Protein estimation: Protein concentration of enzyme solutions was determined by Folin-Lowry method [25] using BSA as a standard. Absorbance was measured at 640 nm in UV Spectrophotometer (Shimadzu UV 1800). Protein bound [%] to the polymer was determined on the basis of the residual unbound protein in the filtrate.
Copolymer characterization
Particle size and surface morphology: Particle size distribution of non-saponified poly(VAc-co-DVB) copolymer beads with different CLD was determined by laser diffraction analyzer, HELOS H1004 and was found to be mainly in the range of 300-500 μm. (Figure 1). With increasing CLD, particle size distribution increases in the range of 300-500 μm while it decreases in the range of 100-300 μm, though there is marginal difference. This shift towards larger particles with increasing CLD may be due to aggregation of droplets with increasing polymerization rate owing to increase in molar concentrations of crosslinking agent and these aggregated droplets seeming grown together.
Figure 1: Particle size distribution of non-saponified poly(VAc-co-DVB) copolymer beads [blue bars indicate particles < 50 μm, red bars indicate particles in the range of 50-100 μm, green bars indicate particles in the range of 100-300 μm, purple bars indicate particles in the range of 300-500 μm and orange bars indicate particles > 500 μm]. (intended for color reproduction on the Web (free of charge) and in print).
SEM micrographs indicate the smooth spherical nature of the polymer beads (Figure 2 100X resolution).
Surface area and pore size distribution: Besides the surface properties of polymer particles, the major factors that contribute to the immobilization of enzyme are pore volume and pore size distribution, which influence the diffusion of substrate and product during the biocatalytic reactions. Mercury porosimetry provides a good estimate of pore size and pore size distribution in the meso- and macroporous region. As per IUPAC notation, Pores with a diameter <2 nm are called micropores, those with a diameter between 2 nm to 50 nm are called mesopores and those with a diameter >50 nm are called macropores. Macropores are more advantageous than meso-micropores, because macropores reduce diffusional limitations between substrate molecules and enzyme during biocatalysis, resulting in increased reaction rate. Pore architecture is also governed by various factors including pore generating solvent ratio, continuous phase and the rate of polymerization, which leads to the formation of various pore types namely open pores, closed pores, ink-pot pores, cylindrical pores.
Surface area and pore size distribution of non-saponified poly(VAcco- DVB) copolymer beads were estimated using mercury porosimetry and porosity data is summarized in Table 2. Pearson correlation coefficient between CLD and surface area is 0.348 which indicates a poor, positive correlation. Surface area of copolymer beads with different CLD was found to increase in CLD 25% to 75%, thereafter it decreased marginally (Figure 3). Maximum surface area (204.45 m2/g) was found in PVAc-DVB-75-UH copolymer beads. Vaidya et al. [26] reported that surface area of poly(GMA-co-EGDM) and poly(AGEco- EGDM) copolymer beads increased with increasing CLD from 50% to 200%. The increase in surface area with cross-link density is due to a decrease in the microporosity. The effect of CLD on Surface area is well reported, however the effect is relatively influenced by monomer composition, type and volume of pore generating solvents and the process conditions leading to pore architecture and hence the effects varies with individual systems.
| Polymer | Average pore diameter (nm) | Surface area (m2/g) | Pore volume (cc/g) | ||
|---|---|---|---|---|---|
| Micropores | Mesopores | Macropores | |||
| PVAc-DVB-25-UH | 78 | 89.66 | 0 | 0.33594 | 1.49737 |
| PVAc-DVB-50-UH | 115 | 131.35 | 0 | 0.4337 | 0.99552 |
| PVAc-DVB-75-UH | 88 | 204.45 | 0 | 0.60926 | 1.03457 |
| PVAc-DVB-100-UH | 80.5 | 181.00 | 0 | 0.3475 | 0.6585 |
| PVAc-DVB-150-UH | 70 | 182.04 | 0 | 0.5454 | 0.75398 |
| PVAc-DVB-200-UH | 68 | 140.84 | 0 | 0.46872 | 0.6285 |
Table 2: Porosity data of poly(VAc-co-DVB) copolymer beads.
Pearson correlation coefficient between CLD and pore diameter is -0.625 which indicates a good, negative correlation implying that with increased CLD, pore diameter decreases (Table 2) With increasing CLD from 50% to 200%, an average pore diameter is decreased from 115 nm to 69 nm (Figure 3).
Distribution of pore diameters of non-saponified poly(VAc-co- DVB) copolymer beads is graphically depicted in Figure 4. None of the copolymers have micropores. This is advantageous for immobilized enzyme in that micropores generally create diffusional limitations because of small size. Correlation coefficient between% CLD and volume of mesopores is 0.34 (a poor, positive correlation) and that of between% CLD and volume of macropores is -0.82 (a very good, negative correlation). This is expected in that with increasing CLD, cross-link network in the bead increases. Percentage of mesopores has increased in CLD 25% to 100%; thereafter it is decreased for CLD 150% and 200%. Percentage of macropores is found to be decreased with increase in CLD. PVAc-DVB-50-UH has maximum percentage of macropores amongst all the copolymers.
Figure 4: Pore size distribution of non-saponified poly(VAc-co-DVB) copolymer beads. [blue bars indicate micropores, red bars indicate mesopores and green bars indicate macropores; All the polymers showed maximum macropores and none of them showed micropores].(intended for color reproduction on the Web (free of charge) and in print).
Immobilization studies
Immobilization of CALB on non-saponified poly(VAc-co- DVB) copolymer beads: Based on the literature available so far, this is the first report on using poly(VAc-co-DVB) copolymer beads being directly used for CALB enzyme immobilization with stable activity. Effect of varying cross-link densities of non-saponified poly(VAc-co- DVB) copolymer beads on immobilization of CALB was shown in Table 3. Correlation coefficient between CLD and Activity (TBU/g dry) of immobilized copolymers is -0.528 indicating fairly good negative correlation. The protein binding (%) and activity was found to increase initially from CLD 25% to CLD 75%, thereafter both were found to decline with increase in CLD. This can be attributed to increasing surface area from CLD 25% to CLD 75%, thereafter decreasing across CLD 100% to 200%. Maximum activity expression shown in PVAc- DVB-50-UH amongst all the copolymers can be explained by its macroporous nature. PVAc-DVB-50-UH has maximum percentage of macropores amongst all the copolymers. Macroporous polymer structures primarily facilitate the easy diffusion of globular enzyme molecules inside the pores thereby enhancing the interaction of the latter with the reactive groups of the polymer. Besides this, presence of macroporous structures effectively reduces the diffusional limitations (of substrate as well as product) during biocatalytic reactions thereby accelerating rate of reaction which ultimately leads to enhanced catalytic expression of the immobilized enzyme [27,28].
| CALB immobilized copolymer beads | Lewatit VP OC 1600 | PVAc-DVB-25- UH | PVAc-DVB-50-UH | PVAc-DVB-75-UH | PVAc-DVB-100-UH | PVAc-DVB-150-UH | PVAc-DVB-200-UH |
|---|---|---|---|---|---|---|---|
| Activitya | 1804 | 984 | 1873 | 2051 | 1373 | 1486 | 1392 |
| Activityb | 2405.33 | 1782.61 | 2647.35 | 2610.41 | 1755.31 | 1744.13 | 1635.53 |
| % protein binding | 70.80 | 74.8 | 74.15 | 78.0 | 68.84 | 74.97 | 72.26 |
| % activity expression | 25.82 | 22.66 | 31.76 | 27.31 | 18.31 | 19.13 | 18.71 |
a TBU/g of wet CALB immobilized copolymer beads
b TBU/g of dry CALB immobilized copolymer beads
where One TBU unit of enzyme activity was defined as the amount of enzyme catalyzing the formation of 1 μmmol of free fatty acid from 10 % (v/v) tributyrin in the milieu of 100 mM sodium phosphate buffer at pH 7.5 and at 40°C.
Table 3: Immobilization of CALB on non-saponified poly(VAc-co-DVB) copolymer beads.
CALB immobilized PVAc-DVB-50-UH beads have 10% higher activity (2647.35 TBU/g dry beads) as compared to that of Lewatit VP OC 1600 (2405.33 TBU/g dry beads). Though the specific surface area of both these polymer beads were similar at 130 m2/g dry beads, there was a significant difference in the pore size, with PVAc-DVB-50-UH showing a pore size of 115 nm as against the pore size of 15 nm in Lewatit VP OC 1600 [16]. However, the difference in activity of both the polymer beads was just 10%. This may be due to the difference of surface interaction of both the polymers with enzyme in the presence of buffer during immobilization as observed by Handayani et al. [18]. In the case of Lewatit VP OC 1600, polymer is composed of poly(methyl methacrylate-co-divinylbenzene) while PVAc-co-DVB polymer is composed of poly(vinyl acetate-co-divinyl benzene). Due to this compositional difference, the enzyme is expected to behave differently. Handayani et al. reported the use of highly macroporous copolymer beads of poly(GMA-co-EGDM) with varying pore diameter, specific surface area and specific volume in the immobilization of CALB. Based on the results, they proposed two reasons for poor loading of the enzyme namely: (1) only the inner surface of mesopores were used for the attachment of enzyme (2) attached enzyme molecule could exert a steric hindrance against other enzymes molecules penetrating into deeper part of the polymer. So, in case of PVAc-DVB-50-UH, bound enzyme molecules could exert a steric hindrance against other enzymes molecules to enter the pores, resulting in decreased activity even if pore size is large (115 nm).
Vaidya et al. [26] compared poly(AGE-co-EGDM) and poly(GMAco- EGDM) copolymer beads for immobilization of L-aminoacylase and found that AGE copolymer beads gave better immobilization of L-aminoacylase than GMA copolymer beads. This is due to the higher specific surface area of AGE copolymer beads than that of GMA copolymer beads. Again, AGE copolymer beads contain large percentage of meso-and macropores in contrast to GMA copolymer beads which typically contain large percentage of micropores.
Li et al. [29] observed that the distribution of Burkholderia cepacia lipase on the polystyrene microsphere depended on the pore sizes. The lipase could penetrate into the centre of the polymer beads, especially in the case of gigaporous (> 200 nm) and macroporous beads and on contrary, the binding was only on the surface in the case of mesoporous and microporous microspheres. As discussed before, the maximum enzyme activity obtained in CALB immobilized PVAc-DVB-50-UH could be explained by the prevalence of more macropores (69.71%) in combination with higher surface area (130 m2/g).
Since there were no reports on any polymer supports with VAc functionality binding enzyme directly without any modification, it was intriguing to know the possible mechanism of enzyme binding. Since there is overall hydrophobicity on the poly(VAc-co-DVB) copolymer due to the pendent VAc groups, the enzyme binding could be expected as physical adsorption via interfacial activation as known earlier in the literature [19-21]. Porous polymeric matrices with great internal active and dense layers of hydrophobic groups can be easily recognized by lipases, at the molecular level, as solid surfaces. Thus, lipases can be adsorbed on such supports resulting in open and stabilized form of immobilized lipases. VAc is a preferred substrate for lipase [30,31]; the presence of pendent VAc group in the beads may be better recognized as a substrate as well as a hydrophobic surface for anchoring.
Immobilization of CALB on saponified poly(VAc-co-DVB) copolymer beads: Effect of saponification of poly(VAc-co-DVB) copolymer beads on CALB immobilization was shown in Table 4. In case of CALB immobilized poly(VAc-co-DVB) copolymer beads with lower CLD (25% to 75%), enzyme activity and activity expression of non-saponified beads were higher than that of saponified beads. However, at higher CLD, enzyme activity and activity expression of non-saponified beads were lower than saponified beads. This difference in activity expression of saponified and non-saponified poly(VAc-co- DVB) copolymer beads could be related to hydrophobic/hydrophilic balance of the polymer beads. Hydrophobic/hydrophilic balance of the polymer varies with its functional and surface active groups and this balance has profound influence on enzyme immobilization, especially CALB. Poly(VAc-co-DVB) copolymer beads offer relative hydrophobicity due to the presence of pendent acetate groups. During the process of partial saponification, some of the pendent vinyl acetate groups get hydrolyzed into vinyl alcohol, thus providing hydroxyl surface chemistry for enzyme binding. Thus non-saponified poly(VAc-co-DVB) beads offers hydrophobicity which favours CALB binding whereas in case of saponified beads, there will be a shift in the hydrophobic/hydrophilic balance towards hydrophillicity, depending on degree of hydrolysis.
| CALB immobilized copolymer beads | Lewatit VP OC 1600 | PVAc-DVB-25-8H | PVAc-DVB-50-8H | PVAc-DVB-75-8H | PVAc-DVB-100-8H | PVAc-DVB-150-8H | PVAc-DVB-200-8H |
|---|---|---|---|---|---|---|---|
| Activitya | 1804 | 549 | 1013 | 1353 | 1462 | 1517 | 1503 |
| Activityb | 2405.33 | 1167.34 | 1706.54 | 1858.0 | 1622.64 | 1820.47 | 1856.70 |
| % protein binding | 70.80 | 84.48 | 82.26 | 70.74 | 73.28 | 75.68 | 76.17 |
| % activity expression | 25.82 | 19.85 | 22.22 | 23.26 | 21.84 | 21.39 | 21.77 |
aTBU/g of wet CALB immobilized copolymer beads
bTBU/g of dry CALB immobilized copolymer beads
where One TBU unit of enzyme activity was defined as the amount of enzyme catalyzing the formation of 1 μmmol of free fatty acid from 10 % (v/v) tributyrin in the milieu of 100 mM sodium phosphate buffer at pH 7.5 and at 40°C.
Table 4: Immobilization of CALB on poly(VAc-co-DVB) copolymer beads saponified for 8 h.
Galarneau et al. [32] studied the binding of two lipases from Mucor miehei on various supports with varying degree of hydrophobic/ hydrophilic nature. Too hydrophilic (pure silica) or too hydrophobic (butyl-grafted silica) supports are not appropriate to develop high activity for lipases. An adequate hydrophobic/hydrophilic balance of the support, such as supported-micelle, provides the best route to enhance lipase activity.Sponge Mesoporous Silicas (SMS), synthesized from the mixture of lecithin and amines in a sol-gel synthesis offered a new support with highest activity for the lipases. The lecithin/amines mixture structuring the pore network leads to a suitable phospholipids bilayer-like environment, with proper hydrophobic/hydrophilic balance. Reis et al. [33] studied the binding of R. miehei lipase at the three different surfaces: one entirely hydrophobic, one hydrophobic but containing carboxyl groups, and one hydrophilic, in which the two hydrophobic surfaces bind much more lipase than the hydrophilic surface. The surface that contains carboxyl groups binds slightly more than the surface with only terminal methyl groups. The low value of adsorption at the hydroxyl-functional surface is expected since hydrophilic surfaces in general are known to be less protein adsorbing than hydrophobic surfaces.
Storage stability of CALB immobilized copolymer beads
CALB immobilized copolymer beads with maximum activity (PVAc-DVB-50-UH) and CALB immobilized Lewatit VP OC 1600 copolymer beads were analyzed for storage stability for 100 days. Storage stability data is summarized in Table 5. Residual enzyme activity of CALB immobilized PVAc-DVB-50-UH and Lewatit VP OC 1600 copolymer beads after 100 days of storage at 5°C was determined by tributyrin hydrolysis assay and found to be 94.82% and 96.05% respectively. Though, the stability of CALB immobilized on Lewatit VP OC 1600 is marginally better than PVAc-DVB-50UH, the overall stability establishes comparable performance. (Pearson coefficient is 0.963) One of the objectives of the study is to develop and evaluate an alternative CALB enzyme as compared to commercially available enzymes and our results corroborate with the same.
| Day of analysis | Activity (TBU/g dry) of CALB immobilized Lewatit VP OC 1600 copolymer beads | % residual activity | Activity (TBU/g dry) of CALB immobilized PVAc-DVB-50-UH copolymer beads | % residual activity |
|---|---|---|---|---|
| Day 1 | 2405.33 | 100 | 2647.35 | 100 |
| Day 20 | 2400.23 | 99.79 | 2625.40 | 99.17 |
| Day 35 | 2356.86 | 97.98 | 2600.12 | 98.22 |
| Day 50 | 2335.62 | 97.10 | 2575.32 | 97.28 |
| Day 65 | 2325.30 | 96.67 | 2555.36 | 96.53 |
| Day 80 | 2315.86 | 96.28 | 2535.20 | 95.76 |
| Day 100 | 2310.42 | 96.05 | 2510.22 | 94.82 |
% residual activity was determined by considering activity at day 1 as 100% by a formula.
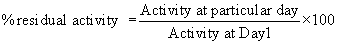
Table 5: Storage stabilitya data of immobilized Candida antarctica lipase B catalysts (CALB).
The use of immobilized enzyme, especially in new applications are expanding and so it is imperative to have novel methods and supports for enzyme immobilization. Considering the growing potential of immobilized CALB enzyme catalysts, there is a need for the development of novel polymer systems with comparable characteristics of commercially available enzymes. Based on the results of the present study, we conclude that a potential new polymer support in the form of macroporous poly(vinyl acetate-co-divinyl benzene) copolymer beads is available for CALB lipase immobilization and subsequent application. The immobilized enzyme on this new support showed good CALB activity and storage stability.
Authors are grateful to all R&D lab colleagues, especially to Ms. Trupti Ashar and Mr. Krishna Muralidharan and the management team of Fermenta Biotech Ltd for their coordination and support.