Journal of Physical Chemistry & Biophysics
Open Access
ISSN: 2161-0398
ISSN: 2161-0398
Research Article - (2017) Volume 7, Issue 3
A signal crystal of NaGd(PO3)4 (NGP) was successfully grown with the solid-state reaction method under appropriate synthesis conditions. Structure refinements and the purity of the samples were determined by XRD patterns. It reveals that NaGd(PO3)4 compound crystallizes in the monoclinic system with P 21/n space group and with cell parameters a=7.174(1) Å, b=13.033(2) Å, c=9.781(1) Å, β=90.65(2)°, V=914.47(20) Å3 and Z=4. The IR and Raman spectra also indicated that the phosphoric polyhedra of NaGd(PO3)4 has a acyclic symmetry. Differential thermal analysis is given. This compound is thermally stable until 852°C. The magnetic susceptibilities of gadolinium polyphosphate as a function of temperature are reported along with corresponding magnetization measurements. This polyphosphate displays a paramagnetic response, without magnetic phase transitions, and with effective magnetic moments close to those of the corresponding free Gd3+ ions. The luminescence proprieties of Gd3+ was investigated. The emission spectra NGP exhibit intense band at 315 nm due to 6P7/2 →8S7/2 transition of Gd3+ ions when excited at 273 nm. The photon cascade emission (PCE) of Gd3+ has been proved at 164 and 254 nm excitation.
<Keywords: Photon cascade emission; Polyphosphates; Radiation
The condensed polyphosphates MLn(PO3)4 with interesting physical properties in various fields are relatively stable in normal conditions of temperature, humidity [1,2] and they are not water soluble [3]. The great structural variety of rare earth polyphosphates provides compounds with very interesting optical proprieties [4-8]. Laser materials is the most important application of those phosphates [9], which was discovered in neodymium phosphates NdP5O14 and LiNdP4O12 [10,11]. Polyphosphates of rare earth are considered as inorganic compounds, which can used as luminophore, these materials have the distinction to emit visible radiation when it is submitted to excitation sources [12]. Recently, NaLa(PO3)4 is considered as an excellent host compound for luminescence of lanthanide ions. The NaGd(PO3)4:Ce3+ compound can be used as scintillator materials [13,14]. Gd3+ ions possess strong excitation band at about 273 nm due to 8S7/2→6IJ transitions [15,16] and gives the emission at 315 nm. This particular beam has got important applications especially in the treatment of various skin diseases [17]. Gd3+ ions do not absorb or emit radiation in the IR or VIS ranges of the electromagnetic spectrum [18].
The condensed phosphates NaGd(PO3)4 were prepared by the solid-state reaction. They starting reactive are Na2CO3, Gd2O3 and NH4H2PO4. Raw materials are used with the following stoichiometry coefficients M=2.1 (M=alkali metal), RE=1 (RE=rare earth metal) and P=8. Firstly, the mixture is milled for one hour or more to homogenize the solid phase, in order to improve the inter atomic diffusion. Second, we will be using an adequate thermal program with different levels. The first levels at 430°C to eliminate H2O, NH3 and CO2, the second one at 730°C to get NaGd(PO3)4 pure after that we will cool at a rate of 2°C/min for good crystallinity of products. The obtained crystals are washed with warm water and nitric acid to eliminate the remaining Gd2O3 oxide.
The samples were characterized using a INEL XRG 3000 (D5000T) diffractometre with monochromatic Cu Kα radiation. The diffraction pattern was recorded under 300 K over the angular range 10-90° (2Ɵ). The luminescence spectra were performed under ambient atmosphere via Xenius (the fluorescence Genius) spectrophotometer, excitation and emission spectra with 254 nm, 164 and 273 nm respectively. The infrared spectrum were recorded in the 250–1500 cm-1 range with a Thermo SCCIENTIFIC NICOLET i N10 MX using sample dispersed in KBr pellets. Raman spectrum was carried out at room temperature, with the 514.5 nm radiation from an argon ion laser as the excitation beam. A microscope allowed selection of a region of good optical quality in the crystalline sample. The thermal stability of NGP was measured with differential thermal analysis SETARAM TAG 16 operating from room temperature up to 1000°C at an average rate of 5°C min-1 and the magnetic measurements were carried out using Quantum Design MPMSXL magnetometer with detection SQUID (at institute NEEL France).
Crystal structural aspects
NaGd(PO3)4 sample prepared at high temperatures under 730°C would show colorless, transparent and parallelepiped-shaped. The morphology of obtaining polyphosphates crystals is shown in Figure 1. The sample prepared by solid state-reactions is stable in air and its crystallized into a monoclinic system, the XRD patterns of sodium gadolinium polyphosphate were indexed using Fullprof [19], from which the Bragg diffraction positions was confirms the crystal structure obtained by the SHELXL-97 program [20] as shown in Figure 2. The samples were of single-phase purity because no extra peak that corresponds to secondary phases or impurity was observed. The crystal structure of NaGd(PO3)4 can be described as three-dimensional framework in which (PO3)44 − chains are linked together with Na+ and Gd3+ ions (Figure 3). The Na+ cation is coordinated by four oxygen atoms with the Na+-O2- bond distances from 2.369 (4) to 2.986 (4) Å. Eight oxygen atoms with Gd3+-O2- bond distances ranging from 2.340(3) to 2.467(3) Å, coordinate the Gd3+ cation. Furthermore, the NaO4 tetrahedra and GdO8 dodecahedra are alternately arranged via edge sharing along b-axis.
Raman and IR
The analysis of the Raman spectra is based on the comparison with homologous compounds [21-23] and at the same time with the calculated frequencies of the chain (PO3)44 −. From Figure 4 we can be simply observed that NaGd(PO3)4 Raman spectra is dominated by two well and intense bands at 696 cm-1 and 1184 cm-1, they are assigned respectively, to the symmetric stretching vibration modes of (P-O-P) and (O-P-O)-. Those bands are characteristic of long chain polyphosphates, so we can therefore confirm that NaGd(PO3)4 crystallize in chain structure. In the low frequency below then 655 cm-1 is very difficult to distingue the lines of deformation δ (PO2) and δ (POP).
The IR spectra is shown in Figure 5. All the band are assigned by comparison with Li(x)Na(1-x)Sm(PO3)4(x=0, 0.5, 1) [24]. The most strong IR bands observed in the regions of 1226 and 918 cm-1 are attributed respectively to the asymmetric stretching vibrations ʋas (PO2)- spices and ʋas (POP) bridges. The bands observed in the region 1200-1000 cm-1 and 800-675 cm-1are attributed to the symmetric stretching vibration ʋas (PO2)- and ʋs (POP).
Thermal behaviour
The thermal behavior of NaGd(PO3)4 obtained by differential thermal analyses (DTA) between 25 and 1000°C is displayed in Figure 6. ATD makes it possible to appreciate the purity and good crystallinity of the compound. The ATD curve shows a single endothermic peak around 852°C, which is it the fusion pic of NaGd(PO3)4. Therefore, we can conclude that the sample is monophasic, and stable under normal conditions of temperature and in a good state of crystallization.
Magnetic properties
The magnetic susceptibility and inverse magnetic susceptibility versus temperature for NaGd(PO3)4 are shown in Figure 7. The polyphosphate compound tested gave a paramagnetic response.
The temperature dependence of the inverse of susceptibility χ-1 for high temperatures is given by the following formula:
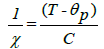
Where ƟP is the Weiss temperature and C is the Curie constant given by:
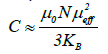
Where N is the number of carriers of magnetic moment, μ0 is the vacuum permeability, KB the Boltzmann constant, μB the Bohr magnetron and μeff the effective moment of the carriers. The structure of the samples consists of one magnetic species (i), possessing each a magnetic moment μeff (i), the magnetic susceptibility is given by the relation
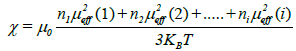
Generally, the magnetic moment is determined by the following equation:
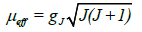
where gJ is the Lande factor and J is the total angular moment.
The theoretical effective paramagnetic moment μexpeff for the samples can be calculated by the following equation:
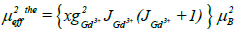
The χ-1 curves allow deducing the values of μexpeff . All the results are given in Table 1. We can notice that the values of μexpeff are equal to the magnetic moment of Gd3+ ions (7.94 μB) [25]. When the theoretical effective moment was compared to the experimental one, we can see that the theoretical value are more important than those obtained experimentally. This can be associated to the matrix, which would increase the disorder. Increasing the temperature of the polyphosphate NGP induced thermal agitation, which cause disorientation of the magnetic moments of atoms. So, the decrease of paramagnetism can be clearly observed.
| C (µB.KT-1) | μexpeff(μB) | μexpeff(μB) | |
|---|---|---|---|
| NaGd(PO3)4 | 0.67 | 7.94 | 2.32 |
Table 1:Values of C, μexpeff(μB), μexpeff(μB) for the NaGd(PO3)4 compound.
Luminescence spectroscopy
The Gd3+ ions present a special interest among lanthanides, because its lowest excited 4f levels, 6PJ (J=3/2, 5/2, 7/2), are about 32,000 cm-1 higher than the ground 4f state 8S7/2 [26]. The optical emission spectrum of Gd3+ ions in NaGd(PO3)4 is shown in Figure 8. The spectrum of NaGd(PO3)4 consists of one absorption band of low intensity originating from internal 4f–4f transitions [27]. The peak is well resolved and very intense that facilitates the assignment. The observed peak under 273 nm excitations is assigned to the typical 6P7/2→ 8S7/2 emission around 315 nm. Figure 9 shows the emission spectra of NaGd(PO3)4 with λex=164 nm and λex=254 nm. Several groups of sharp lines are observed which can be attributed to 6GJ→6PJ around 690 nm and 6PJ→6IJ around (763 nm).
According to the emission spectra of NGP, photon cascade emission (PCE) of Gd3+ is detected [28-30]. When the electrons are excited to the 6GJ states, three emission processes from PCE of Gd3+. First, The Gd3+ ions radiatively return to intermediate states 6PJ with an emission wavelength around 690 nm. Second, the Gd3+ radiatively return to intermediate states 6IJ and emit a second type of photons with a wavelength 763 nm. At the end, Gd3+ ions in 6PJ sates de-excite to the ground state 8S7/2 and emit a third type of photons with a wavelength around 315 nm [31].
This works describes the synthesis of a sodium polyphosphate compound NaGd(PO3)4 by a solid state-reaction. The single crystal structure has been determined. It crystalizes in the monoclinic system with P 21/n space group and with cell parameters a=7.1740(10) Å, b=13.033(2) Å, c=9.7810(10) Å, β=90.56(2)°, V=914.5(2) Å3 and Z=4. Infrared and Raman spectroscopy has been investigated as a function of temperature, it shows the existence of bands between 750 and 1000 cm-1 confirms the presence of an infinite chain of PO4 linked by a bridge oxygen in this structure. The thermal behavior of NGP has been followed by ATD. It shows one endothermic peak at 853°C, due to the decomposition of the polyphosphate. In addition, the magnetic measurements of gadolinium polyphosphate reported here display a paramagnetic response, which indicates that there is no interaction between the rare-earth ions. The luminescence spectra of the glasses exhibited intense UVB band at about 315 nm due to 6P7/2→ 8S7/2 transition of Gd3+ ion.